|
Calcium Aluminate Cements
Calcium aluminate cements are cements consisting predominantly of hydraulic calcium aluminates. Alternative names are "aluminous cement", "high-alumina cement", and "Ciment fondu" in French. They are used in a number of small-scale, specialized applications. History The method of making cement from limestone () and low-silica bauxite () was patented in France in 1908 by Bied of the Pavin de Lafarge Company. The initial development was as a result of the search for a cement offering sulfate resistance. The cement was known as "Ciment fondu" and "Ciment électro-fondu" in French. As indicated by Bied (1922), who was the inventor of this type of cement, the terms "Ciment fondu" ("fused cement") and "Ciment électro-fondu" ("electro-fused cement") refer only to the manufacturing process involving the melting of the base materials (CaO obtained after the decarbonation of , and ). This is because there is no temperature range in which it is possible to observe the gradual softening ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Silica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundant families of materials, existing as a compound of several minerals and as a synthetic product. Examples include fused quartz, fumed silica, opal, and aerogels. It is used in structural materials, microelectronics, and as components in the food and pharmaceutical industries. All forms are white or colorless, although impure samples can be colored. Silicon dioxide is a common fundamental constituent of glass. Structure In the majority of silicon dioxides, the silicon atom shows Tetrahedral molecular geometry, tetrahedral coordination, with four oxygen atoms surrounding a central Si atomsee 3-D Unit Cell. Thus, SiO2 forms 3-dimensional network solids in which each silicon atom is covalently bonded in a tetrahedral manner to 4 oxygen atoms. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
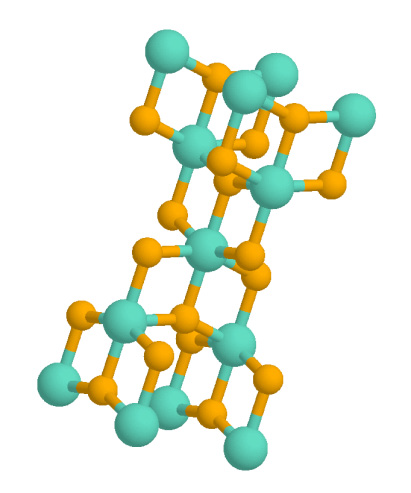
Calcium Aluminoferrite
Calcium aluminoferrite () is a dark brown crystalline phase commonly found in cements. In the cement industry it is termed tetra-calcium aluminoferrite or ferrite. In cement chemist notation (CCN), it is abbreviated as meaning in the oxide notation. It also exists in nature as the rare mineral brownmillerite. Properties of the pure phase In the absence of elements other than calcium, aluminium, iron and oxygen, calcium aluminoferrite forms a solid solution series of formula for all values of x in the range 0–0.7.Taylor H.F.W. (1990). ''Cement Chemistry'', Academic Press, . Compositions with x > 0.7 do not exist at ordinary pressures (see dicalcium aluminate). The crystal is orthorhombic, and is normally lath-like. Its density varies from 4026 kg·m−3 (x = 0) to 3614 kg⋅m−3 (x = 0.7). All compositions melt incongruently in the range 1400−1450 °C. They are ferromagnetic, progressively more so as iron content increases. These phases are easily prepared ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gehlenite
Gehlenite, (Ca2Al lSiO7, is a sorosilicate, Al-rich endmember of the melilite complete solid solution series with akermanite.Deer et al., 1993 The type locality is in the Monzoni Mountains, Fassa Valley in Trentino in Italy, and is named after the Adolf Ferdinand Gehlen (1775–1815) by A.J. Fuchs in 1815.Dana et al. 1997 Geological occurrence Gehlenite is found in carbonaceous chondrites from which it condensed as a refractory mineral in the hotter stages ( FU Ori) of the presolar nebula,Grossman L (1972) Condensation in the primitive solar nebula, Geochemica et Cosmochemica Acta, 36, 597–619 and was subsequently consumed in processes which created enstatite and other more abundant minerals making it a remnant mineral from the early solar nebula (along with corundum and spinel). Its occurrence in the early condensation phase of the solar nebula was predicted by Harry Lord in the 1950s, but studies of carbonaceous chondrites did not support this claim until the Allende mete ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Belite
Belite is an industrial mineral important in Portland cement manufacture. Its main constituent is dicalcium silicate, Ca2SiO4, sometimes formulated as 2 CaO · SiO2, SiO2 (C2S in cement chemist notation). Etymology The name was given by Alfred Elis Törnebohm in 1897 to a crystal identified in microscopic investigation of Portland cement. Belite is a name in common use in the cement industry, but is not a recognised mineral name. It occurs naturally as the mineral larnite, the name being derived from Larne, Northern Ireland, the closest town to Scawt Hill where it was discovered. Composition and structure The belite found in Portland cement differs in composition from pure dicalcium silicate. It is a solid solution and contains minor amounts of other oxides besides CaO and SiO2. A typical composition:Taylor H.F.W. (1990), ''Cement Chemistry'', Academic Press, 1990, , pp. 10-11. Based on this, the formula can be expressed as Ca1.94Mg0.02Na0.01K0.03Fe0.02Al0.07Si0.90P0.01O ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dodecacalcium Hepta-aluminate
Dodecacalcium hepta-aluminate (12CaO·7Al2O3, Ca12Al14O33 or ) is an inorganic solid that occurs rarely in nature as the mineral mayenite. It is an important phase in calcium aluminate cements and is an intermediate in the manufacture of Portland cement. Its composition and properties have been the subject of much debate, because of variations in composition that can arise during its high-temperature formation.Taylor, H F W (1990) ''Cement Chemistry'', Academic Press, , pp. 36–38 Synthesis Polycrystalline can be prepared via a conventional solid-state reaction, i.e., heating a mixture of calcium carbonate and aluminium oxide or aluminium hydroxide powders, in air. It is not formed in oxygen or in moisture-free atmosphere. It can be regrown into single crystals using the Czochralski or zone melting techniques. In Portland cement kilns, is an early reaction product of aluminium and calcium oxides in the temperature range 900–1200 °C. With the onset of melt-phases at hi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Monocalcium Aluminate
Monocalcium aluminate (CaAl2O4) is one of the series of calcium aluminates. It does occur in nature, although only very rarely, as two polymorphs known as krotite and dmitryivanovite, both from meteorites. It is important in the composition of calcium aluminate cements. Properties Monocalcium aluminate is formed when the appropriate proportions of calcium carbonate and aluminium oxide are heated together until the mixture melts. It melts incongruently at 1390 °C. The crystal is monoclinic and pseudohexagonal, and has density 2945 kg·m−3. In calcium aluminate cements, it exists as a solid solution in which the amount of minor elements depends upon the bulk composition of the cement A cement is a binder, a chemical substance used for construction that sets, hardens, and adheres to other materials to bind them together. Cement is seldom used on its own, but rather to bind sand and gravel ( aggregate) together. Cement mi .... A typical compositionP. C. H ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Titanium Dioxide
Titanium dioxide, also known as titanium(IV) oxide or titania , is the inorganic compound derived from titanium with the chemical formula . When used as a pigment, it is called titanium white, Pigment White 6 (PW6), or Colour Index International, CI 77891. It is a white solid that is insoluble in water, although mineral forms can appear black. As a pigment, it has a wide range of applications, including paint, sunscreen, and food coloring. When used as a food coloring, it has E number E171. World production in 2014 exceeded 9 million tonnes. It has been estimated that titanium dioxide is used in two-thirds of all pigments, and pigments based on the oxide have been valued at a price of $13.2 billion. Structure In all three of its main dioxides, titanium exhibits Octahedral molecular geometry, octahedral geometry, being bonded to six oxide anions. The oxides in turn are bonded to three Ti centers. The overall crystal structures of rutile and anatase are tetragonal in symmetry ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Potassium Oxide
Potassium oxide ( K O) is an ionic compound of potassium and oxygen. It is a base. This pale yellow solid is the simplest oxide of potassium. It is a highly reactive compound that is rarely encountered. Some industrial materials, such as fertilizers and cements, are assayed assuming the percent composition that would be equivalent to K2O. Production Potassium oxide is produced from the reaction of oxygen and potassium; this reaction affords potassium peroxide, K2O2. Treatment of the peroxide with potassium produces the oxide: : Alternatively and more conveniently, K2O is synthesized by heating potassium nitrate with metallic potassium: : Other possibility is to heat potassium peroxide at 500 °C which decomposes at that temperature giving pure potassium oxide and oxygen. : Potassium hydroxide cannot be further dehydrated to the oxide but it can react with molten potassium to produce it, releasing hydrogen as a byproduct. : Properties and reactions K2O crystallises ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sodium Oxide
Sodium oxide is a chemical compound with the formula . It is used in ceramics and glasses. It is a white solid but the compound is rarely encountered. Instead "sodium oxide" is used to describe components of various materials such as glasses and fertilizers which contain oxides that include sodium and other elements. Sodium oxide is a component. Structure The structure of sodium oxide has been determined by X-ray crystallography. Most alkali metal oxides (M = Li, Na, K, Rb) crystallise in the antifluorite structure. In this motif the positions of the anions and cations are reversed relative to their positions in , with sodium ions tetrahedrally coordinated to 4 oxide ions and oxide cubically coordinated to 8 sodium ions. Preparation Sodium oxide is produced by the reaction of sodium with sodium hydroxide, sodium peroxide, or sodium nitrite: : To the extent that NaOH is contaminated with water, correspondingly greater amounts of sodium are employed. Excess sodium is disti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium Oxide
Magnesium oxide (MgO), or magnesia, is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium (see also oxide). It has an empirical formula of MgO and consists of a lattice of Mg2+ ions and O2− ions held together by ionic bonding. Magnesium hydroxide forms in the presence of water (MgO + H2O → Mg(OH)2), but it can be reversed by heating it to remove moisture. Magnesium oxide was historically known as magnesia alba (literally, the white mineral from Magnesia), to differentiate it from '' magnesia nigra'', a black mineral containing what is now known as manganese. Related oxides While "magnesium oxide" normally refers to MgO, the compound magnesium peroxide MgO2 is also known. According to evolutionary crystal structure prediction, MgO2 is thermodynamically stable at pressures above 116 GPa (gigapascals), and a semiconducting suboxide Mg3O2 is thermodynamically stable above 500 GPa. Because of its stability, MgO is used as a mod ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |