|
Caesium Clock Block
Caesium (IUPAC spelling; also spelled cesium in American English) is a chemical element; it has Symbol (chemistry), symbol Cs and atomic number 55. It is a soft, silvery-golden alkali metal with a melting point of , which makes it one of only five elemental metals that are liquid at or near room temperature. Caesium has physical and chemical properties similar to those of rubidium and potassium. It is pyrophoricity, pyrophoric and reacts with water even at . It is the least electronegativity, electronegative stable element, with a value of 0.79 on the Pauling scale. It has only one stable isotope, caesium-133. Caesium is mined mostly from pollucite. Caesium-137, a fission product, is extracted from waste produced by nuclear reactor technology, nuclear reactors. It has the largest atomic radius of all elements whose radii have been measured or calculated, at about 260 picometres. The German chemist Robert Bunsen and physicist Gustav Kirchhoff discovered caesium in 1860 by the new ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
IUPAC
The International Union of Pure and Applied Chemistry (IUPAC ) is an international federation of National Adhering Organizations working for the advancement of the chemical sciences, especially by developing nomenclature and terminology. It is a member of the International Science Council (ISC). IUPAC is registered in Zürich, Switzerland, and the administrative office, known as the "IUPAC Secretariat", is in Research Triangle Park, North Carolina, United States. IUPAC's executive director heads this administrative office, currently Greta Heydenrych. IUPAC was established in 1919 as the successor of the International Congress of Applied Chemistry for the advancement of chemistry. Its members, the National Adhering Organizations, can be national List of chemistry societies, chemistry societies, national Academy of Sciences, academies of sciences, or other bodies representing chemists. There are fifty-four National Adhering Organizations and three Associate National Adhering Organ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pollucite
Pollucite is a zeolite mineral with the formula with iron, calcium, rubidium and potassium as common substituting elements. It is important as a significant ore of caesium and sometimes rubidium. It forms a solid solution series with analcime. It crystallizes in the isometric-hexoctahedral crystal system as colorless, white, gray, or rarely pink and blue masses. Well-formed crystals are rare. It has a Mohs hardness of 6.5 and a specific gravity of 2.9, with a brittle fracture and no cleavage. Discovery and occurrence It was first described by August Breithaupt in 1846 for occurrences on the island of Elba, Italy. It is named for Pollux, the twin of Castor on the grounds that it is often found associated with petalite (previously known as ''castorite''). The high caesium content was missed by the first analysis by Karl Friedrich Plattner in 1848, but after the discovery of caesium in 1860 a second analysis in 1864 was able to show the high caesium content of pollucite. Its t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
International System Of Units
The International System of Units, internationally known by the abbreviation SI (from French ), is the modern form of the metric system and the world's most widely used system of measurement. It is the only system of measurement with official status in nearly every country in the world, employed in science, technology, industry, and everyday commerce. The SI system is coordinated by the International Bureau of Weights and Measures, which is abbreviated BIPM from . The SI comprises a coherent system of units of measurement starting with seven base units, which are the second (symbol s, the unit of time), metre (m, length), kilogram (kg, mass), ampere (A, electric current), kelvin (K, thermodynamic temperature), mole (mol, amount of substance), and candela (cd, luminous intensity). The system can accommodate coherent units for an unlimited number of additional quantities. These are called coherent derived units, which can always be represented as products of powers of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Clock
An atomic clock is a clock that measures time by monitoring the resonant frequency of atoms. It is based on atoms having different energy levels. Electron states in an atom are associated with different energy levels, and in transitions between such states they interact with a very specific frequency of electromagnetic radiation. This phenomenon serves as the basis for the International System of Units' (SI) definition of a second: The second, symbol s, is the SI unit of time. It is defined by taking the fixed numerical value of the caesium frequency, \Delta \nu_\text, the unperturbed ground-state hyperfine transition frequency of the caesium-133 atom, to be when expressed in the unit Hz, which is equal to s−1. This definition is the basis for the system of International Atomic Time (TAI), which is maintained by an ensemble of atomic clocks around the world. The system of Coordinated Universal Time, Coordinated Universal Time (UTC) that is the basis of civil time implements ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solar Cell
A solar cell, also known as a photovoltaic cell (PV cell), is an electronic device that converts the energy of light directly into electricity by means of the photovoltaic effect.Solar Cells chemistryexplained.com It is a type of photoelectric cell, a device whose electrical characteristics (such as Electric current, current, voltage, or Electrical resistance and conductance, resistance) vary when it is exposed to light. Individual solar cell devices are often the electrical building blocks of solar panel, photovoltaic modules, known colloquially as "solar panels". Almost all commercial PV cells consist of crystalline silicon, with a market share of 95%. Cadmium telluride thin-film solar cells account for the remainder. The common single-junction silicon solar cell can produce a maximum open-circuit voltage o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vacuum Tube
A vacuum tube, electron tube, thermionic valve (British usage), or tube (North America) is a device that controls electric current flow in a high vacuum between electrodes to which an electric voltage, potential difference has been applied. It takes the form of an evacuated tubular envelope of glass or sometimes metal containing electrodes connected to external connection pins. The type known as a thermionic tube or thermionic valve utilizes thermionic emission of electrons from a hot cathode for fundamental Electronics, electronic functions such as signal amplifier, amplification and current Rectifier, rectification. Non-thermionic types such as vacuum phototubes achieve electron emission through the photoelectric effect, and are used for such purposes as the detection of light and measurement of its intensity. In both types the electrons are accelerated from the cathode to the anode by the electric field in the tube. The first, and simplest, vacuum tube, the diode or Flem ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
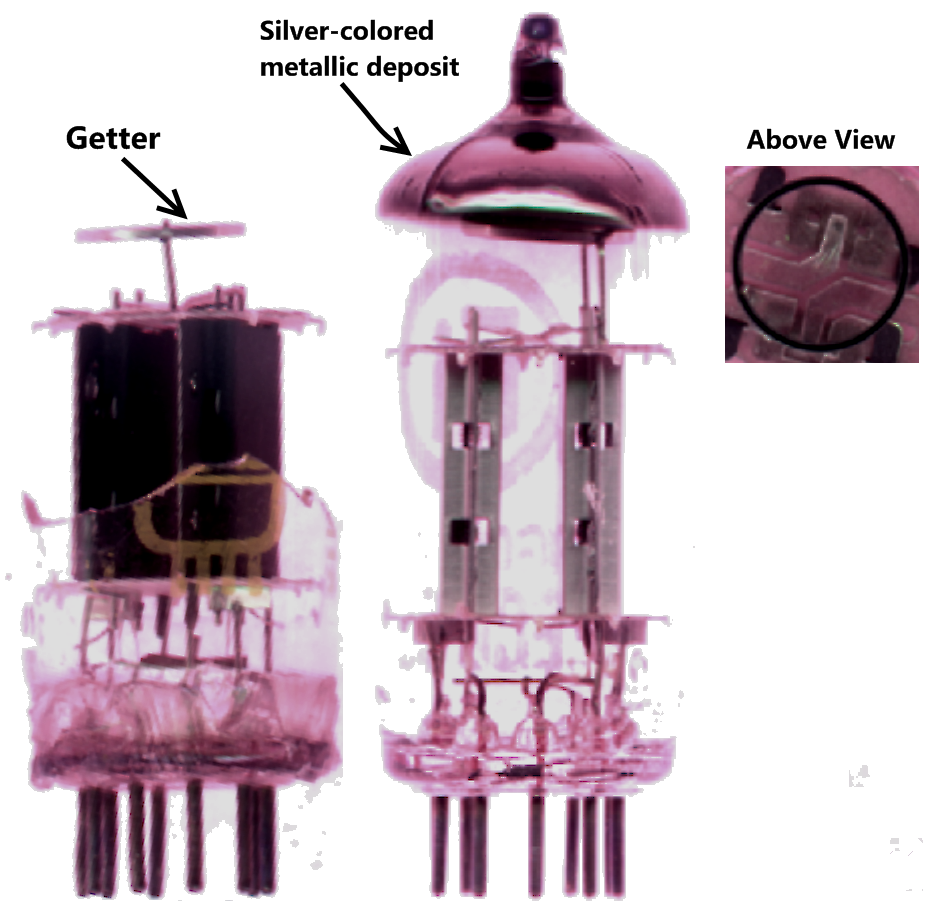
Getter
A getter is a deposit of reactive material that is placed inside a vacuum system to complete and maintain the vacuum. When gas molecules strike the getter material, they combine with it chemically or by adsorption. Thus the getter removes small amounts of gas from the evacuated space. The getter is usually a coating applied to a surface within the evacuated chamber. A vacuum is initially created by connecting a container to a vacuum pump. After achieving a sufficient vacuum, the container can be sealed, or the vacuum pump can be left running. Getters are especially important in sealed systems, such as vacuum tubes, including cathode-ray tubes (CRTs), vacuum insulating glass (or vacuum glass) and vacuum insulated panels, which must maintain a vacuum for a long time. This is because the inner surfaces of the container release adsorbed gases for a long time after the vacuum is established. The getter continually removes residues of a reactive gas, such as oxygen, as long as it i ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Emission Spectroscopy
Atomic emission spectroscopy (AES) is a method of chemical analysis that uses the intensity of light emitted from a flame, plasma, arc, or spark at a particular wavelength to determine the quantity of an element in a sample. The wavelength of the atomic spectral line in the emission spectrum gives the identity of the element while the intensity of the emitted light is proportional to the number of atoms of the element. The sample may be excited by various methods. Atomic Emission Spectroscopy allows us to measure interactions between electromagnetic radiation and physical atoms and molecules. This interaction is measured in the form of electromagnetic waves representing the changes in energy between atomic energy levels. When elements are burned by a flame, they emit electromagnetic radiation that can be recorded in the form of spectral lines. Each element has its own unique spectral line because each element has a different atomic arrangement, so this method is an important ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Gustav Kirchhoff
Gustav Robert Kirchhoff (; 12 March 1824 – 17 October 1887) was a German chemist, mathematician, physicist, and spectroscopist who contributed to the fundamental understanding of electrical circuits, spectroscopy and the emission of black-body radiation by heated objects. He also coined the term ''black body'' in 1860. Several different sets of concepts are named "Kirchhoff's laws" after him, which include Kirchhoff's circuit laws, Kirchhoff's law of thermal radiation, and Kirchhoff's law of thermochemistry. The Bunsen–Kirchhoff Award for spectroscopy is named after Kirchhoff and his colleague, Robert Bunsen. Life and work Gustav Kirchhoff was born on 12 March 1824 in Königsberg, Prussia, the son of Friedrich Kirchhoff, a lawyer, and Johanna Henriette Wittke. His family were Lutheranism, Lutherans in the Evangelical Church of Prussia. He graduated from the Albertus University of Königsberg in 1847 where he attended the mathematico-physical seminar directed by Carl Gusta ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Robert Bunsen
Robert Wilhelm Eberhard Bunsen (; 30 March 1811 – 16 August 1899) was a German chemist. He investigated emission spectra of heated elements, and discovered caesium (in 1860) and rubidium (in 1861) with the physicist Gustav Kirchhoff. The Bunsen–Kirchhoff Award for spectroscopy is named after Bunsen and Kirchhoff. Bunsen also developed several gas-analytical methods, was a pioneer in photochemistry, and did early work in the field of organic arsenic chemistry. With his laboratory assistant Peter Desaga, he developed the Bunsen burner, an improvement on the laboratory burners then in use. Early life and education Bunsen was born in Göttingen, Germany, in 1811, in what is now the state of Lower Saxony in Germany. Bunsen was the youngest of four sons of the University of Göttingen's chief librarian and professor of modern philology, Christian Bunsen (1770–1837). After attending school in Holzminden, Bunsen matriculated at Göttingen in 1828 and studied chemistry wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Picometre
The picometre (international spelling as used by the International Bureau of Weights and Measures; SI symbol: pm) or picometer (American spelling) is a unit of length in the International System of Units (SI), equal to , or one trillionth of a metre, which is the SI base unit of length. The picometre is one thousand femtometres, one thousandth of a nanometre ( nm), one millionth of a micrometre (also known as a micron), one billionth of a millimetre, and one trillionth of a metre. The symbol μμ was once used for it. It is also one hundredth of an ångström, an internationally known (but non-SI) unit of length. Use The picometre's length is of an order so small that its application is almost entirely confined to particle physics, quantum physics, chemistry, and acoustics. Atoms are between 62 and 520 pm in diameter, and the typical length of a carbon–carbon single bond is 154 pm. Smaller units still may be used to describe smaller particles (some of which ar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Atomic Radius
The atomic radius of a chemical element is a measure of the size of its atom, usually the mean or typical distance from the center of the nucleus to the outermost isolated electron. Since the boundary is not a well-defined physical entity, there are various non-equivalent definitions of atomic radius. Four widely used definitions of atomic radius are: Van der Waals radius, ionic radius, metallic radius and covalent radius. Typically, because of the difficulty to isolate atoms in order to measure their radii separately, atomic radius is measured in a chemically bonded state; however theoretical calculations are simpler when considering atoms in isolation. The dependencies on environment, probe, and state lead to a multiplicity of definitions. Depending on the definition, the term may apply to atoms in condensed matter, covalently bonding in molecules, or in ionized and excited states; and its value may be obtained through experimental measurements, or computed from theore ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |