|
C-8813
C-8813 (thiobromadol) is a potent μ-opioid receptor agonist with a distinctive chemical structure which is not closely related to other established families of opioid drugs. The ''trans''-isomer was found to be around 591 times more potent than morphine in animal studies. The same study assigned a potency of 504 times that of morphine to the related compound BDPC. C-8813 is claimed to be similarly potent at the δ-opioid receptor, which antagonizes the μ-induced depression of breathing, presumably making the drug safer. C-8813 has never been approved for use in humans. See also * BDPC * Ciramadol * Faxeladol * Profadol * Tapentadol * Tramadol Tramadol, sold under the brand name Tramal among others, is an opioid analgesic, pain medication and a serotonin–norepinephrine reuptake inhibitor (SNRI) used to treat moderately severe pain. When taken by mouth in an immediate-release form ... References Arylcyclohexylamines Synthetic opioids Thiophenes Tert ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Synthetic Opioids
Opioids are a class of Drug, drugs that derive from, or mimic, natural substances found in the Papaver somniferum, opium poppy plant. Opioids work on opioid receptors in the brain and other organs to produce a variety of morphine-like effects, including analgesic, pain relief. The terms "opioid" and "opiate" are sometimes used interchangeably, but the term "opioid" is used to designate all substances, both natural and synthetic, that bind to opioid receptors in the brain. Opiates are alkaloid compounds naturally found in the opium poppy plant ''Papaver somniferum''. Medically they are primarily used for pain relief, including anesthesia. Other medical uses include suppression of diarrhea, replacement therapy for opioid use disorder, and Cold medicine, suppressing cough. The opioid receptor antagonist naloxone is used to reverse opioid overdose. Extremely potent opioids such as carfentanil are approved only for Veterinary medicine, veterinary use. Opioids are also frequently use ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
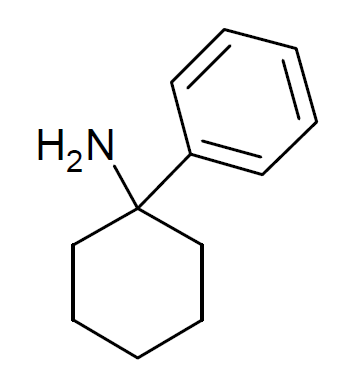
Arylcyclohexylamines
Arylcyclohexylamines, also known as arylcyclohexamines or arylcyclohexanamines, are a chemical class of pharmaceutical drug, pharmaceutical, designer drug, designer, and experimental drugs. History Phencyclidine (PCP) is believed to be the first arylcyclohexylamine with recognized anesthetic properties, but several arylcyclohexylamines were described before PCP in the scientific literature, beginning with PCA (1-phenylcyclohexan-1-amine) the synthesis of which was first published in 1907. PCP itself was discovered in 1926 but not researched by the pharmaceutical industry until the 1950s. Eticyclidine, PCE was reported in 1953 and PCMo (4-(1-phenyl-cyclohexyl)-morpholine see chart below for figure) in 1954, with PCMo described as a potent sedative. Arylcyclohexylamine anesthetics were intensively investigated at Parke-Davis, beginning with the 1956 studies of PCP and later the related compound ketamine. The 1970s saw the debut of these compounds, especially PCP and its structura ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
BDPC
BDPC (systematic name 4-(4-bromophenyl)-4-(dimethylamino)-1-(2-phenylethyl)cyclohexanol; also known as bromadol) is a potent fully synthetic opioid with a distinctive arylcyclohexylamine chemical structure. It was developed by Daniel Lednicer at Upjohn in the 1970s. Initial studies estimated that it was around 10,000 times the potency of morphine in animal models. However, later studies using more modern techniques assigned a value of 504 times the potency of morphine for the more active ''trans''-isomer. This drug was first seized along with three kilograms of acetylfentanyl in an April 25, 2013 police action in Montreal, Canada, and has reportedly continued to be available on the designer drug market internationally. Analogues where the ''para''-bromine is replaced by chlorine or a methyl group retain similar activity, while the ''meta''-hydroxyl derivative demonstrated robust antagonist activity. ] ] See also * 3-HO-PCP * 4-Keto-PCP * C-8813 * Cebranopadol * Ciramadol * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ciramadol
Ciramadol (WY-15,705) is an opioid analgesic that was developed in the late 1970s and is related to phencyclidine, tramadol, tapentadol and venlafaxine. It is a mixed agonist-antagonist for the μ-opioid receptor with relatively low abuse potential and a ceiling on respiratory depression which makes it a relatively safe drug. It has a slightly higher potency and effectiveness as an analgesic than codeine, but is weaker than morphine. Other side effects include sedation and nausea but these are generally less severe than with other similar drugs. Synthesis The Claisen-Schmidt condensation between 3-(methoxymethoxy)benzaldehyde 3709-05-2(1) and cyclohexanone (2) affordeCID:54364197(3). Michael addition of dimethylamine Dimethylamine is an organic compound with the formula (CH3)2NH. This secondary amine is a colorless, flammable gas with an ammonia-like odor. Dimethylamine is commonly encountered commercially as a solution in water at concentrations up to around ... leads t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Profadol
Profadol (CI-572) is an opioid analgesic which was developed in the 1960s by Parke-Davis. It acts as a mixed agonist-antagonist of the μ-opioid receptor. The analgetic potency is about the same as of pethidine (meperidine), the antagonistic effect is 1/50 of nalorphine. Synthesis The Knoevenagel condensation between 3'-Methoxybutyrophenone 1550-06-1and Ethyl cyanoacetate gives (1). Conjugate addition of cyanide gives (2). Hydrolysis of both nitrile groups, saponification of the ester and decarboxylation gives the diacidCID:164137621(3). Imide formation occurs upon treatment with methylamine giving 3-(3-Methoxyphenyl)-1-methyl-3-propylpyrrolidine-2,5-dioneCID:163444474(4). Reduction of the imide by lithium aluminium hydride gave 505-32-429369-01-5] (5). Demethylation completed the synthesis of Profadol (6). See also * BDPC, Bromadol * C-8813 * Ciramadol * Faxeladol * Prodilidine * Tapentadol * Tramadol Tramadol, sold under the brand name Tramal among others, is ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Agonist
An agonist is a chemical that activates a Receptor (biochemistry), receptor to produce a biological response. Receptors are Cell (biology), cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an Receptor antagonist, antagonist blocks the action of the agonist, while an inverse agonist causes an action opposite to that of the agonist. Etymology The word originates from the Ancient Greek, Greek word (''agōnistēs''), "contestant; champion; rival" < (''agōn''), "contest, combat; exertion, struggle" < (''agō''), "I lead, lead towards, conduct; drive." Types of agonists Receptor (biochemistry), Receptors can be activated by either endogenous agonists (such as hormones and neurotransmitters) or exogenous agonists (such as medication, drugs), resulting in a biological response. A physiological agonism an ...[...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Morphine
Morphine, formerly also called morphia, is an opiate that is found naturally in opium, a dark brown resin produced by drying the latex of opium poppies (''Papaver somniferum''). It is mainly used as an analgesic (pain medication). There are multiple methods used to administer morphine: oral; sublingual administration, sublingual; via inhalation; intramuscular, injection into a muscle, Subcutaneous injection, injection under the skin, or injection into the spinal cord area; transdermal; or via rectal administration, rectal suppository. It acts directly on the central nervous system (CNS) to induce analgesia and alter perception and emotional response to pain. Physical and psychological dependence and tolerance may develop with repeated administration. It can be taken for both acute pain and chronic pain and is frequently used for pain from myocardial infarction, kidney stones, and during Childbirth, labor. Its maximum effect is reached after about 20 minutes when administ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
δ-opioid Receptor
The δ-opioid receptor, also known as delta opioid receptor or simply delta receptor, abbreviated DOR or DOP, is an inhibitory 7-transmembrane G-protein coupled receptor coupled to the G protein Gi alpha subunit, Gi/G0 and has enkephalins as its endogenous ligands. The regions of the brain where the δ-opioid receptor is largely expressed vary from species model to species model. In humans, the δ-opioid receptor is most heavily expressed in the basal ganglia and Neocortex, neocortical regions of the brain. Function The endogenous system of opioid receptors is well known for its analgesic potential; however, the exact role of δ-opioid receptor activation in pain modulation is largely up for debate. This also depends on the model at hand since receptor activity is known to change from species to species. Activation of delta receptors produces analgesia, perhaps as significant potentiators of μ-opioid receptor agonists. However, it seems like delta agonism provides heavy poten ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Faxeladol
Faxeladol (INN, USAN) (code names GRTA-9906, GRTA-0009906, EM-906, GCR-9905, GRT-TA300) is an opioid analgesic which was developed by Grünenthal GmbH but was never marketed for medical use anywhere in the world. It is related to tramadol and ciramadol, and was developed shortly after tramadol in the late 1970s. Similarly to tramadol, it was believed faxeladol would have analgesic, as well as antidepressant effects, due to its action on serotonin and norepinephrine reuptake. In various studies in the 1970s alongside tramadol, faxeladol was seen to be slightly more potent than tramadol, but with a higher rate of sudden seizures than tramadol, which is known to cause seizures without warning in some users. See also * Bromadol * Profadol Profadol (CI-572) is an opioid analgesic which was developed in the 1960s by Parke-Davis. It acts as a mixed agonist-antagonist of the μ-opioid receptor. The analgetic potency is about the same as of pethidine (meperidine), the antagonistic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tapentadol
Tapentadol, sold under the brand names Nucynta and Palexia among others, is a synthetic opioid analgesic with a dual mode of action as a highly selective full agonist of the μ-opioid receptor and as a norepinephrine reuptake inhibitor (NRI). Tapentadol is used medically for the treatment of moderate to severe pain. It is highly addictive and is a commonly abused drug. Common side effects include euphoria, constipation, nausea, vomiting, headaches, loss of appetite, drowsiness, dizziness, itching, dry mouth, and sweating. Serious side effects may include addiction and dependence, substance abuse, respiratory depression and an increased risk of serotonin syndrome. Combining tapentadol with certain substances, including serotonergic drugs or other central nervous system depressants such as alcohol, cannabis, benzodiazepines, and other opioids, may increase the risk of serotonin syndrome, sedation, respiratory depression, and death. Analgesia occurs within 32 minutes of oral ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tramadol
Tramadol, sold under the brand name Tramal among others, is an opioid analgesic, pain medication and a serotonin–norepinephrine reuptake inhibitor (SNRI) used to treat moderately severe pain. When taken by mouth in an immediate-release formulation, the onset of pain relief usually begins within an hour. It is also available by injection. It is available in combination with paracetamol (acetaminophen). As is typical of opioids, common side effects include constipation, pruritis, itchiness, and nausea. Serious side effects may include hallucinations, seizures, increased risk of serotonin syndrome, decreased alertness, and drug addiction. A change in dosage may be recommended in those with chronic kidney disease, kidney or liver problems. It is not recommended in those who are at risk of suicide or in those who are pregnant. While not recommended in women who are breastfeeding, those who take a single dose should not generally have to stop breastfeeding. Tramadol is converted ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |