|
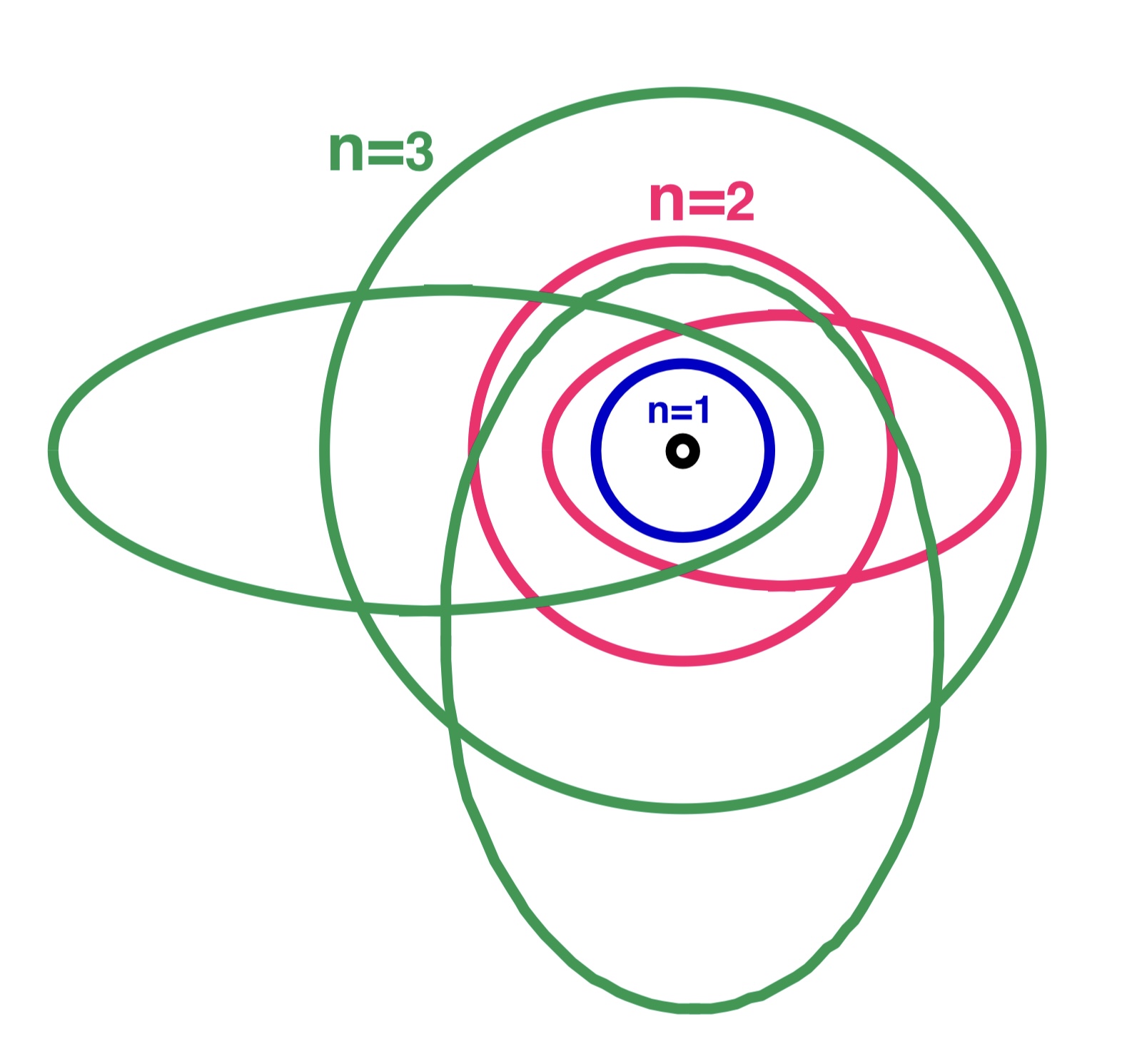
Bohr–Sommerfeld Model
The Bohr–Sommerfeld model (also known as the Sommerfeld model or Bohr–Sommerfeld theory) was an extension of the Bohr model to allow elliptical orbits of electrons around an atomic nucleus. Bohr–Sommerfeld theory is named after Danish physicist Niels Bohr and German physicist Arnold Sommerfeld. Sommerfeld argued that if electronic orbits could be elliptical instead of circular, the energy of the electron would be the same, except in the presence of a magnetic field, introducing what is now known as quantum degeneracy. The Bohr–Sommerfeld model supplemented the quantized angular momentum condition of the Bohr model with an additional radial quantization condition, the Wilson–Sommerfeld quantization condition : \int_0^T p_r \,dq_r = n h, where ''pr'' is the radial momentum canonically conjugate to the coordinate ''q'', which is the radial position, and ''T'' is one full orbital period. The integral is the action of action-angle coordinates. This condition, sugge ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Drawing Of Sommerfeld Atom
Drawing is a form of visual art in which an artist uses instruments to mark paper or other two-dimensional surface. Drawing instruments include graphite pencils, pen and ink, various kinds of paints, inked brushes, colored pencils, crayons, charcoal, chalk, pastels, erasers, markers, styluses, and metals (such as silverpoint). Digital drawing is the act of drawing on graphics software in a computer. Common methods of digital drawing include a stylus or finger on a touchscreen device, stylus- or finger-to-touchpad, or in some cases, a mouse. There are many digital art programs and devices. A drawing instrument releases a small amount of material onto a surface, leaving a visible mark. The most common support for drawing is paper, although other materials, such as cardboard, wood, plastic, leather, canvas, and board, have been used. Temporary drawings may be made on a blackboard or whiteboard. Drawing has been a popular and fundamental means of public expression throu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zeeman Effect
The Zeeman effect (; ) is the effect of splitting of a spectral line into several components in the presence of a static magnetic field. It is named after the Dutch physicist Pieter Zeeman, who discovered it in 1896 and received a Nobel prize for this discovery. It is analogous to the Stark effect, the splitting of a spectral line into several components in the presence of an electric field. Also similar to the Stark effect, transitions between different components have, in general, different intensities, with some being entirely forbidden (in the dipole approximation), as governed by the selection rules. Since the distance between the Zeeman sub-levels is a function of magnetic field strength, this effect can be used to measure magnetic field strength, e.g. that of the Sun and other stars or in laboratory plasmas. The Zeeman effect is very important in applications such as nuclear magnetic resonance spectroscopy, electron spin resonance spectroscopy, magnetic resonance ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Matrix Mechanics
Matrix mechanics is a formulation of quantum mechanics created by Werner Heisenberg, Max Born, and Pascual Jordan in 1925. It was the first conceptually autonomous and logically consistent formulation of quantum mechanics. Its account of quantum jumps supplanted the Bohr model's electron orbits. It did so by interpreting the physical properties of particles as matrices that evolve in time. It is equivalent to the Schrödinger wave formulation of quantum mechanics, as manifest in Dirac's bra–ket notation. In some contrast to the wave formulation, it produces spectra of (mostly energy) operators by purely algebraic, ladder operator methods. Relying on these methods, Wolfgang Pauli derived the hydrogen atom spectrum in 1926, before the development of wave mechanics. Development of matrix mechanics In 1925, Werner Heisenberg, Max Born, and Pascual Jordan formulated the matrix mechanics representation of quantum mechanics. Epiphany at Helgoland In 1925 Werner Heisenberg was ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Wolfgang Pauli
Wolfgang Ernst Pauli (; ; 25 April 1900 – 15 December 1958) was an Austrian theoretical physicist and one of the pioneers of quantum physics. In 1945, after having been nominated by Albert Einstein, Pauli received the Nobel Prize in Physics for his "decisive contribution through his discovery of a new law of Nature, the exclusion principle or Pauli principle". The discovery involved spin theory, which is the basis of a theory of the structure of matter. Early years Pauli was born in Vienna to a chemist, Wolfgang Joseph Pauli (''né'' Wolf Pascheles, 1869–1955), and his wife, Bertha Camilla Schütz; his sister was Hertha Pauli, a writer and actress. Pauli's middle name was given in honor of his godfather, physicist Ernst Mach. Pauli's paternal grandparents were from prominent families of Prague; his great-grandfather was the publisher Wolf Pascheles. Pauli's mother, Bertha Schütz, was raised in her mother's Roman Catholic religion; Pauli was raised as a Roman Cathol ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnetic Quantum Number
In atomic physics, the magnetic quantum number () is one of the four quantum numbers (the other three being the principal, azimuthal, and spin) which describe the unique quantum state of an electron. The magnetic quantum number distinguishes the orbitals available within a subshell, and is used to calculate the azimuthal component of the orientation of orbital in space. Electrons in a particular subshell (such as s, p, d, or f) are defined by values of (0, 1, 2, or 3). The value of can range from to , including zero. Thus the s, p, d, and f subshells contain 1, 3, 5, and 7 orbitals each, with values of within the ranges 0, ±1, ±2, ±3 respectively. Each of these orbitals can accommodate up to two electrons (with opposite spins), forming the basis of the periodic table. Derivation There is a set of quantum numbers associated with the energy states of the atom. The four quantum numbers n, \ell, m_\ell, and s specify the complete quantum state of a single electron in an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Walther Kossel
Walther Ludwig Julius Kossel (4 January 1888 – 22 May 1956) was a German physicist known for his theory of the chemical bond (ionic bond/octet rule), Sommerfeld–Kossel displacement law of atomic spectra, the Kossel-Stranski model for crystal growth, and the Kossel effect. Walther was the son of Albrecht Kossel who won the Nobel Prize in Physiology or Medicine in 1910. Career Kossel was born in Berlin, and began studies at the University of Heidelberg in 1906, but was at the University of Berlin during 1907 and 1908. In 1910, he became assistant to Philipp Lenard, who was also his thesis advisor. Kossel was awarded his Ph.D. in 1910, and he stayed on as assistant to Leonard until 1913. In 1913, the year in which Niels Bohr introduced the Bohr model of the atom, Kossel went to the University of Munich as assistant to Arnold Sommerfeld, under whom he did his Habilitation. Under Sommerfeld, Munich was a theoretical center for the developing atomic theory, especially from t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnetic Field
A magnetic field is a vector field that describes the magnetic influence on moving electric charges, electric currents, and magnetic materials. A moving charge in a magnetic field experiences a force perpendicular to its own velocity and to the magnetic field. A permanent magnet's magnetic field pulls on ferromagnetic materials such as iron, and attracts or repels other magnets. In addition, a nonuniform magnetic field exerts minuscule forces on "nonmagnetic" materials by three other magnetic effects: paramagnetism, diamagnetism, and antiferromagnetism, although these forces are usually so small they can only be detected by laboratory equipment. Magnetic fields surround magnetized materials, and are created by electric currents such as those used in electromagnets, and by electric fields varying in time. Since both strength and direction of a magnetic field may vary with location, it is described mathematically by a function assigning a vector to each point of space ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Energy Levels
A quantum mechanical system or particle that is bound—that is, confined spatially—can only take on certain discrete values of energy, called energy levels. This contrasts with classical particles, which can have any amount of energy. The term is commonly used for the energy levels of the electrons in atoms, ions, or molecules, which are bound by the electric field of the nucleus, but can also refer to energy levels of nuclei or vibrational or rotational energy levels in molecules. The energy spectrum of a system with such discrete energy levels is said to be quantized. In chemistry and atomic physics, an electron shell, or principal energy level, may be thought of as the orbit of one or more electrons around an atom's nucleus. The closest shell to the nucleus is called the " shell" (also called "K shell"), followed by the " shell" (or "L shell"), then the " shell" (or "M shell"), and so on farther and farther from the nucleus. The shells correspond with the principal ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Stern–Gerlach Experiment
The Stern–Gerlach experiment demonstrated that the spatial orientation of angular momentum is quantized. Thus an atomic-scale system was shown to have intrinsically quantum properties. In the original experiment, silver atoms were sent through a spatially varying magnetic field, which deflected them before they struck a detector screen, such as a glass slide. Particles with non-zero magnetic moment are deflected, due to the magnetic field gradient, from a straight path. The screen reveals discrete points of accumulation, rather than a continuous distribution, owing to their quantized spin. Historically, this experiment was decisive in convincing physicists of the reality of angular-momentum quantization in all atomic-scale systems. After its conception by Otto Stern in 1921, the experiment was first successfully conducted by Walther Gerlach in early 1922. Description The Stern–Gerlach experiment involves sending a beam of silver atoms through an inhomogeneous magnetic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Path Integral Formulation
The path integral formulation is a description in quantum mechanics that generalizes the action principle of classical mechanics. It replaces the classical notion of a single, unique classical trajectory for a system with a sum, or functional integral, over an infinity of quantum-mechanically possible trajectories to compute a quantum amplitude. This formulation has proven crucial to the subsequent development of theoretical physics, because manifest Lorentz covariance (time and space components of quantities enter equations in the same way) is easier to achieve than in the operator formalism of canonical quantization. Unlike previous methods, the path integral allows one to easily change coordinates between very different canonical descriptions of the same quantum system. Another advantage is that it is in practice easier to guess the correct form of the Lagrangian of a theory, which naturally enters the path integrals (for interactions of a certain type, these are ''coordi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Quantum Chaos
Quantum chaos is a branch of physics which studies how chaotic classical dynamical systems can be described in terms of quantum theory. The primary question that quantum chaos seeks to answer is: "What is the relationship between quantum mechanics and classical chaos?" The correspondence principle states that classical mechanics is the classical limit of quantum mechanics, specifically in the limit as the ratio of Planck's constant to the action of the system tends to zero. If this is true, then there must be quantum mechanisms underlying classical chaos (although this may not be a fruitful way of examining classical chaos). If quantum mechanics does not demonstrate an exponential sensitivity to initial conditions, how can exponential sensitivity to initial conditions arise in classical chaos, which must be the correspondence principle limit of quantum mechanics?''Quantum Signatures of Chaos'', Fritz Haake, Edition: 2, Springer, 2001, , . Michael Berry, "Quantum Chaology", ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Martin Gutzwiller
Martin may refer to: Places * Martin City (other) * Martin County (other) * Martin Township (other) Antarctica * Martin Peninsula, Marie Byrd Land * Port Martin, Adelie Land * Point Martin, South Orkney Islands Australia * Martin, Western Australia * Martin Place, Sydney Caribbean * Martin, Saint-Jean-du-Sud, Haiti, a village in the Sud Department of Haiti Europe * Martin, Croatia, a village in Slavonia, Croatia * Martin, Slovakia, a city * Martín del Río, Aragón, Spain * Martin (Val Poschiavo), Switzerland England * Martin, Hampshire * Martin, Kent * Martin, East Lindsey, Lincolnshire, hamlet and former parish in East Lindsey district * Martin, North Kesteven, village and parish in Lincolnshire in North Kesteven district * Martin Hussingtree, Worcestershire * Martin Mere, a lake in Lancashire ** WWT Martin Mere, a wetland nature reserve that includes the lake and surrounding areas * Martin Mill, Kent North America Canada * Rural Municipality of ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
.jpg)