|
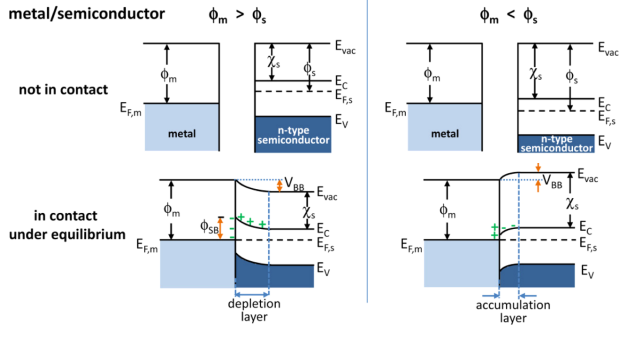
Band Bending
In solid-state physics, band bending refers to the process in which the electronic band structure in a material curves up or down near a junction or interface. It does not involve any physical (spatial) bending. When the electrochemical potential of the free charge carriers around an interface of a semiconductor is dissimilar, charge carriers are transferred between the two materials until an equilibrium state is reached whereby the potential difference vanishes. The band bending concept was first developed in 1938 when Mott, Davidov and Schottky all published theories of the rectifying effect of metal-semiconductor contacts. The use of semiconductor junctions sparked the computer revolution in 1990. Devices such as the diode, the transistor, the photocell and many more still play an important role in technology. Qualitative description Band bending can be induced by several types of contact. In this section metal-semiconductor contact, surface state, applied bias and adsorption ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Solid-state Physics
Solid-state physics is the study of rigid matter, or solids, through methods such as quantum mechanics, crystallography, electromagnetism, and metallurgy. It is the largest branch of condensed matter physics. Solid-state physics studies how the large-scale properties of solid materials result from their atomic-scale properties. Thus, solid-state physics forms a theoretical basis of materials science. It also has direct applications, for example in the technology of transistors and semiconductors. Background Solid materials are formed from densely packed atoms, which interact intensely. These interactions produce the mechanical (e.g. hardness and elasticity), thermal, electrical, magnetic and optical properties of solids. Depending on the material involved and the conditions in which it was formed, the atoms may be arranged in a regular, geometric pattern (crystalline solids, which include metals and ordinary water ice) or irregularly (an amorphous solid such as c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Work Function
In solid-state physics, the work function (sometimes spelt workfunction) is the minimum thermodynamic work (i.e., energy) needed to remove an electron from a solid to a point in the vacuum immediately outside the solid surface. Here "immediately" means that the final electron position is far from the surface on the atomic scale, but still too close to the solid to be influenced by ambient electric fields in the vacuum. The work function is not a characteristic of a bulk material, but rather a property of the surface of the material (depending on crystal face and contamination). Definition The work function for a given surface is defined by the difference :W = -e\phi - E_, where is the charge of an electron, is the electrostatic potential in the vacuum nearby the surface, and is the Fermi level (electrochemical potential of electrons) inside the material. The term is the energy of an electron at rest in the vacuum nearby the surface. In practice, one directly controls ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dangling Bond
In chemistry, a dangling bond is an unsatisfied valence on an immobilized atom. An atom with a dangling bond is also referred to as an immobilized free radical or an immobilized radical, a reference to its structural and chemical similarity to a free radical. When speaking of a dangling bond, one is generally referring to the state described above, containing one electron and thus leading to a neutrally charged atom. There are also dangling bond defects containing two or no electrons. These are negatively and positively charged respectively. Dangling bonds with two electrons have an energy close to the valence band of the material and those with none have an energy that is closer to the conduction band. Properties In order to gain enough electrons to fill their valence shells (see also octet rule), many atoms will form covalent bonds with other atoms. In the simplest case, that of a single bond, two atoms each contribute one unpaired electron, and the resulting pair of elect ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lattice (group)
In geometry and group theory, a lattice in the real coordinate space \mathbb^n is an infinite set of points in this space with the properties that coordinate wise addition or subtraction of two points in the lattice produces another lattice point, that the lattice points are all separated by some minimum distance, and that every point in the space is within some maximum distance of a lattice point. Closure under addition and subtraction means that a lattice must be a subgroup of the additive group of the points in the space, and the requirements of minimum and maximum distance can be summarized by saying that a lattice is a Delone set. More abstractly, a lattice can be described as a free abelian group of dimension n which spans the vector space \mathbb^n. For any basis of \mathbb^n, the subgroup of all linear combinations with integer coefficients of the basis vectors forms a lattice, and every lattice can be formed from a basis in this way. A lattice may be viewed as a regu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Surface States
Surface states are electronic states found at the surface of materials. They are formed due to the sharp transition from solid material that ends with a surface and are found only at the atom layers closest to the surface. The termination of a material with a surface leads to a change of the electronic band structure from the bulk material to the vacuum. In the weakened potential at the surface, new electronic states can be formed, so called surface states. Origin at condensed matter interfaces As stated by Bloch's theorem, eigenstates of the single-electron Schrödinger equation with a perfectly periodic potential, a crystal, are Bloch waves : \begin \Psi_ &=\mathrm^u_(\boldsymbol). \end Here u_(\boldsymbol) is a function with the same periodicity as the crystal, ''n'' is the band index and k is the wave number. The allowed wave numbers for a given potential are found by applying the usual Born–von Karman cyclic boundary conditions. The termination of a crystal, i.e. the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Band Bending 2
Band or BAND may refer to: Places *Bánd, a village in Hungary * Band, Iran, a village in Urmia County, West Azerbaijan Province, Iran * Band, Mureș, a commune in Romania * Band-e Majid Khan, a village in Bukan County, West Azerbaijan Province, Iran People * Band (surname), various people with the surname Arts, entertainment, and media Music * Musical ensemble, a group of people who perform instrumental or vocal music **Band (rock and pop), a small ensemble that plays rock or pop ** Concert band, an ensemble of woodwind, brass, and percussion instruments **Dansband, band playing popular music for a partner-dancing audience ** Jazz band, a musical ensemble that plays jazz music **Marching band, a group of instrumental musicians who generally perform outdoors **School band, a group of student musicians who rehearse and perform instrumental music * The Band, a Canadian-American rock and roll group ** ''The Band'' (album), The Band's eponymous 1969 album * "Bands" (song), by Americ ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Accumulation Layer
Accumulation may refer to: Finance * Accumulation function, a mathematical function defined in terms of the ratio future value to present value * Capital accumulation, the gathering of objects of value Science and engineering * Accumulate (higher-order function), a family of functions to analyze a recursive data structure in computer science * Bioaccumulation, of substances, such as pesticides or other chemicals in an organism * Glacier ice accumulation, an element in the glacier mass balance formula * Metabolic trapping, a localization mechanism of the synthesized radiocompounds in human body * Tree accumulation, in computer science, the process of accumulating data placed in tree nodes according to their tree structure * Accumulation point, another name for a limit point * Cumulative sum, for example cumulative distribution function, or cumulative death toll, summarized since start of a catastrophe Other *'' Accumulation: None'', a 2002 lo-fi album See also * * Acc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Depletion Region
In semiconductor physics, the depletion region, also called depletion layer, depletion zone, junction region, space charge region or space charge layer, is an insulating region within a conductive, doped semiconductor material where the mobile charge carriers have been diffused away, or have been forced away by an electric field. The only elements left in the depletion region are ionized donor or acceptor impurities. This region of uncovered positive and negative ions is called the depletion region due to the depletion of carriers in this region. The depletion region is so named because it is formed from a conducting region by removal of all free charge carriers, leaving none to carry a current. Understanding the depletion region is key to explaining modern semiconductor electronics: diodes, bipolar junction transistors, field-effect transistors, and variable capacitance diodes all rely on depletion region phenomena. Formation in a p–n junction A depletion regio ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electric Field
An electric field (sometimes E-field) is the physical field that surrounds electrically charged particles and exerts force on all other charged particles in the field, either attracting or repelling them. It also refers to the physical field for a system of charged particles. Electric fields originate from electric charges and time-varying electric currents. Electric fields and magnetic fields are both manifestations of the electromagnetic field, one of the four fundamental interactions (also called forces) of nature. Electric fields are important in many areas of physics, and are exploited in electrical technology. In atomic physics and chemistry, for instance, the electric field is the attractive force holding the atomic nucleus and electrons together in atoms. It is also the force responsible for chemical bonding between atoms that result in molecules. The electric field is defined as a vector field that associates to each point in space the electrostatic ( Coulomb) for ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrostatic Induction
Electrostatic induction, also known as "electrostatic influence" or simply "influence" in Europe and Latin America, is a redistribution of electric charge in an object that is caused by the influence of nearby charges. In the presence of a charged body, an insulated conductor develops a positive charge on one end and a negative charge on the other end. Induction was discovered by British scientist John Canton in 1753 and Swedish professor Johan Carl Wilcke in 1762. Electrostatic generators, such as the Wimshurst machine, the Van de Graaff generator and the electrophorus, use this principle. See also Stephen Gray in this context. Due to induction, the electrostatic potential (voltage) is constant at any point throughout a conductor. Electrostatic induction is also responsible for the attraction of light nonconductive objects, such as balloons, paper or styrofoam scraps, to static electric charges. Electrostatic induction laws apply in dynamic situations as far as the quasi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
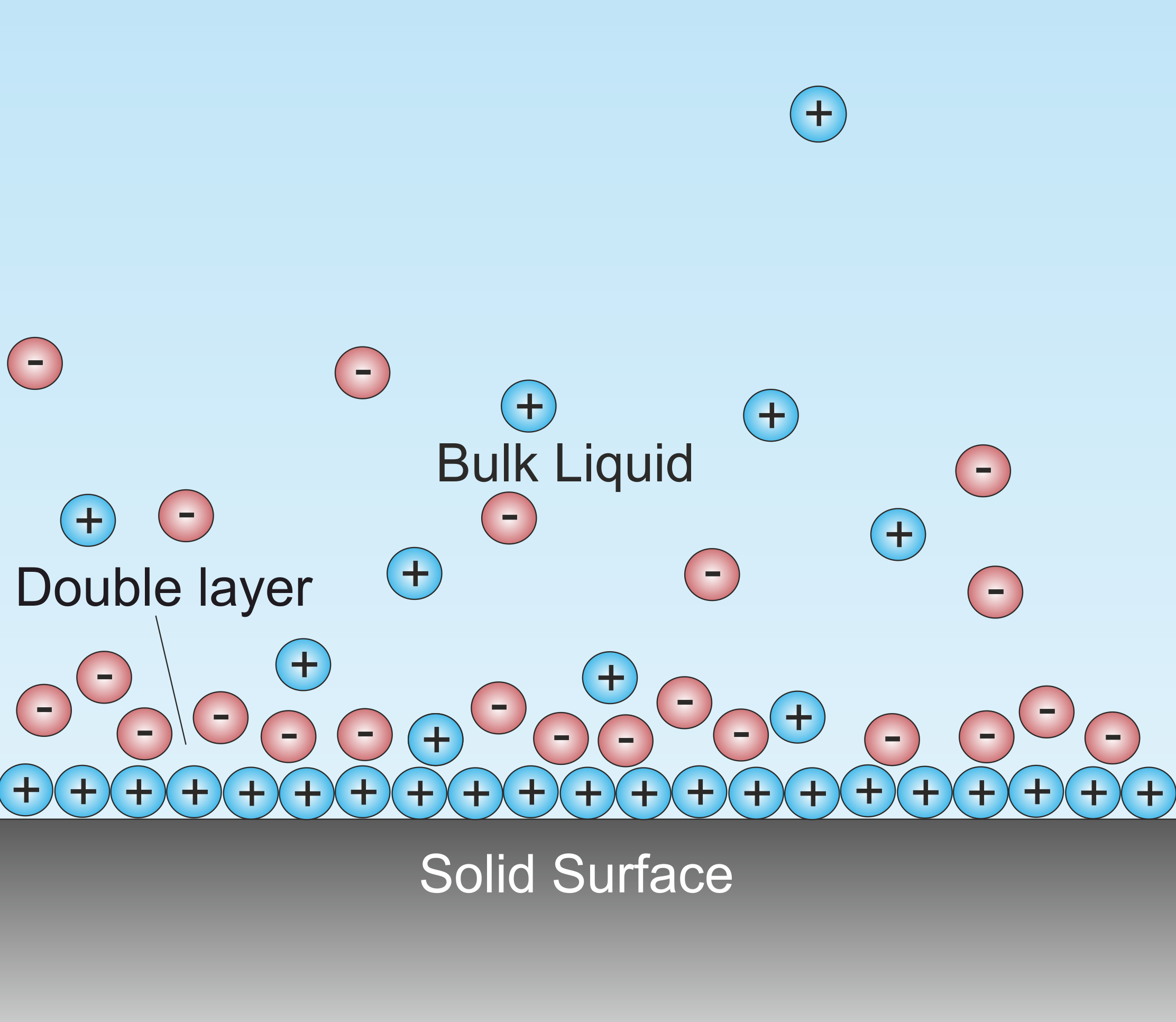
Double Layer (surface Science)
A double layer (DL, also called an electrical double layer, EDL) is a structure that appears on the surface of an object when it is exposed to a fluid. The object might be a solid particle, a gas bubble, a liquid droplet, or a porous body. The DL refers to two parallel layers of charge surrounding the object. The first layer, the surface charge (either positive or negative), consists of ions adsorbed onto the object due to chemical interactions. The second layer is composed of ions attracted to the surface charge via the Coulomb force, electrically screening the first layer. This second layer is loosely associated with the object. It is made of free ions that move in the fluid under the influence of electric attraction and thermal motion rather than being firmly anchored. It is thus called the "diffuse layer". Interfacial DLs are most apparent in systems with a large surface area to volume ratio, such as a colloid or porous bodies with particles or pores (respectively) on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |