|
Bridgewire
A bridgewire or bridge wire, also known as a hot bridge wire (HBW), is a relatively thin resistance wire used to set off a pyrotechnic composition serving as pyrotechnic initiator. By passing of electric current it is heated to a high temperature that starts the exothermic chemical reaction of the attached composition. After successful firing, the bridgewire melts, resulting in an open circuit. Usually a thin nichrome wire is used. Some applications also use platinum-silver alloy; other bridgewire materials in use are platinum, gold, silver, tungsten, etc. Care has to be taken when selecting the material as it is in direct contact with the pyrotechnic composition and should not undergo corrosion in such conditions. Another material, able to actively release chemical energy, is Pyrofuze, aluminium wire clad with palladium; when being heated it undergoes strongly exothermic reaction as the molten metals form an alloy. A variant with the same function consists of laminated thin alt ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Exploding-bridgewire Detonator
The exploding-bridgewire detonator (EBW, also known as exploding wire detonator) is a type of detonator used to initiate the detonation reaction in explosive materials, similar to a blasting cap because it is fired using an electric current. EBWs use a different physical mechanism than blasting caps, using more electricity delivered much more rapidly. They explode with more precise timing after the electric current is applied by the process of exploding wire. The precise timing of exploding wire detonators compared with other types of detonators has led to their common use in nuclear weapons. The slapper detonator is a more recent development along similar lines. History The EBW was invented by Luis Alvarez and Lawrence Johnston for the Fat Man–type bombs of the Manhattan Project, during their work in Los Alamos National Laboratory. The Fat Man Model 1773 EBW detonators used an unusual, high reliability detonator system with two EBW "horns" attached to a single booster ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
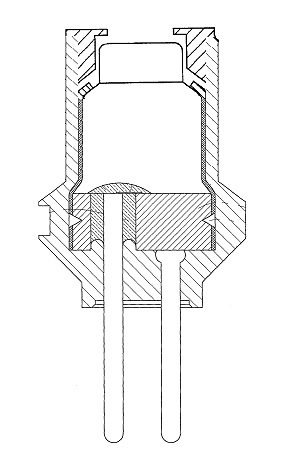
Blasting Cap
A detonator is a device used to make an explosive or explosive device explode. Detonators come in a variety of types, depending on how they are initiated (chemically, mechanically, or electrically) and details of their inner working, which often involve several stages. Types of detonators include non-electric and electric. Non-electric detonators are typically stab or Pyrotechnics, pyrotechnic while electric are typically "hot wire" (low voltage), exploding bridge wire (high voltage) or explosive foil (very high voltage). The original electric detonators invented in 1875 independently by Julius Smith and Perry Gardiner used mercury fulminate as the primary explosive. Around the turn of the century performance was enhanced in the Smith-Gardiner blasting cap by the addition of 10-20% potassium chlorate. This compound was superseded by others: lead azide, lead styphnate, some aluminium, or other materials such as DDNP (diazo dinitro phenol) to reduce the amount of lead emitted in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electric Match
An electric match is a device that uses an externally applied electric current to ignite a combustible compound. Electric matches use a bridgewire consisting of a heating element to ignite a pyrogen, which is a quantity of readily ignited pyrotechnic initiator composition. Electric matches can be used in any application where source of heat is needed at a precisely controlled point in time, typically to ignite a propellant or explosive. Examples include airbags, pyrotechnics, and military or commercial explosives. Design Electric matches consist of two parts, a bridgewire and a Pyrogen (pyrotechnics), pyrogen. The bridgewire is a heating element, typically in the form of a loop or coil of thin wire, which is encased in the pyrogen, which is a quantity of readily ignited pyrotechnic initiator composition. If the pyrogen is sufficiently conductive, it can act as the bridgewire as well. Electric matches also come with provisions for attaching an electric current source, and they ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrotechnic Initiator
In pyrotechnics, a pyrotechnic initiator (also initiator or igniter) is a device containing a pyrotechnic composition used primarily to ignite other, more difficult-to-ignite materials, such as thermites, gas generators, and solid-fuel rockets. The name is often used also for the compositions themselves. Pyrotechnic initiators are often controlled electrically (called electro-pyrotechnic initiators), e.g. using a heated bridgewire or a ''bridge resistor''. They are somewhat similar to blasting caps or other detonators, but they differ in that there is no intention to produce a shock wave. An example of such pyrotechnic initiator is an electric match. Composition The energetic material used, often called pyrogen, is usually a pyrotechnic composition made of a fuel and oxidizer, where the fuel produces a significant amount of hot particles that cause/promote the ignition of the desired material. Initiator compositions are similar to flash powders, but they differ in burning speed, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nichrome
Nichrome (also known as NiCr, nickel-chromium or chromium-nickel) is a family of alloys of nickel and chromium (and occasionally iron) commonly used as resistance wire, heating elements in devices like toasters, electrical kettles and space heaters, in some dental restorations (fillings) and in a few other applications. Patented in 1906 by Albert Marsh (US patent 811,859), nichrome is the oldest documented form of resistance heating alloy. The A Grade nichrome alloy is 80% nickel and 20% chromium by mass, but there are many other combinations of metals for various applications. Properties C Grade Nichrome is consistently silvery in color, is corrosion-resistant, has a high melting point of approximately , and has an electrical resistivity of around 1.12 μΩ·m, which is around 66 times higher resistivity than copper of 16.78 nΩ·m. Some nichrome formulations have a resistivity as low as 1.0 μΩ·m or as high as 1.5 μΩ·m. Almost any conductive w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyrotechnic Fastener
A pyrotechnic fastener (also called an explosive bolt, or pyro, within context) is a fastener, usually a nut or bolt, that incorporates a pyrotechnic charge that can be initiated remotely. One or more explosive charges embedded within the bolt are typically activated by an electric current, and the charge breaks the bolt into two or more pieces. The bolt is typically scored around its circumference at the point(s) where the severance should occur. Such bolts are often used in space applications to ensure separation between rocket stages, because they are lighter and much more reliable than mechanical latches. In applications that require safety, precision and reliability, such as the aerospace industry, pyrotechnic fasteners are triggered using exploding bridgewire detonators, which were themselves later succeeded by slapper detonators. Classical blasting caps are generally avoided for such usage. More recent developments have used pulsed laser diodes to detonate initiators ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Graphite
Graphite () is a Crystallinity, crystalline allotrope (form) of the element carbon. It consists of many stacked Layered materials, layers of graphene, typically in excess of hundreds of layers. Graphite occurs naturally and is the most stable form of carbon under standard conditions. Synthetic and natural graphite are consumed on a large scale (1.3million metric tons per year in 2022) for uses in many critical industries including refractories (50%), lithium-ion batteries (18%), foundries (10%), and lubricants (5%), among others (17%). Graphite converts to diamond under extremely high pressure and temperature. Graphite's low cost, thermal and chemical inertness and characteristic conductivity of heat and electricity finds numerous applications in high energy and high temperature processes. Types and varieties Graphite can occur naturally or be produced synthetically. Natural graphite is obtained from naturally occurring geologic deposits and synthetic graphite is produced t ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Squib (explosive)
A squib is a miniature explosive device used in a wide range of industries, from special effects to military applications. It resembles a tiny stick of dynamite, both in appearance and construction, but has considerably less explosive power. A squib consists of two electrical leads separated by a plug of insulating material; a small bridge wire or electrical resistance heater; and a bead of heat-sensitive chemical composition, in which the bridge wire is embedded. They can be used to generate mechanical force to shatter or propel various materials; and for pyrotechnic effects for film and live theatrics. A squib generally consists of a small tube filled with an explosive substance, with a detonator running through the length of its core, similar to a stick of dynamite. Also similar to dynamite, the detonator can be a slow-burning Fuse (explosives), fuse, or as is more common today, a wire connected to a remote electronics, electronic :wikt:trigger, trigger. Squibs range in s ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Magnesium
Magnesium is a chemical element; it has Symbol (chemistry), symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 of the periodic table), it occurs naturally only in combination with other elements and almost always has an oxidation state of +2. It reacts readily with air to form a thin Passivation (chemistry), passivation coating of magnesium oxide that inhibits further corrosion of the metal. The free metal burns with a brilliant-white light. The metal is obtained mainly by electrolysis of magnesium Salt (chemistry), salts obtained from brine. It is less dense than aluminium and is used primarily as a component in strong and lightweight magnesium alloy, alloys that contain aluminium. In the cosmos, magnesium is produced in large, aging stars by the sequential addition of three Helium nucleus, helium nuclei to a carbon nucleus. When such stars explo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |