|
Biosimilar
A biosimilar (also known as follow-on biologic or subsequent entry biologic) is a biologic medical product that is almost an identical copy of an original product that is manufactured by a different company. Biosimilars are officially approved versions of original "innovator" products and can be manufactured when the original product's patent expires. Reference to the innovator product is an integral component of the approval. Unlike with generic drugs of the more common small-molecule type, biosimilar drugs generally exhibit high molecular complexity and may be quite sensitive to changes in manufacturing processes. Despite this heterogeneity, all biopharmaceuticals, including biosimilars, must maintain consistent quality and clinical performance throughout their lifecycle. Drug-related authorities such as the European Medicines Agency (EMA) of the European Union, the United States Food and Drug Administration (FDA), and the Health Products and Food Branch of Health Canada ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biopharmaceutical
A biopharmaceutical, also known as a biological medical product, or biologic, is any pharmaceutical drug product manufactured in, extracted from, or semisynthesized from biological sources. Different from totally synthesized pharmaceuticals, they include vaccines, whole blood, blood components, allergenics, somatic cells, gene therapies, tissues, recombinant therapeutic protein, and living medicines used in cell therapy. Biopharmaceuticals can be composed of sugars, proteins, nucleic acids, or complex combinations of these substances, or may be living cells or tissues. They (or their precursors or components) are isolated from living sources—human, animal, plant, fungal, or microbial. They can be used in both human and animal medicine. Terminology surrounding biopharmaceuticals varies between groups and entities, with different terms referring to different subsets of therapeutics within the general biopharmaceutical category. The term biologics is often used more res ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Generic Drug
A generic drug is a pharmaceutical drug that contains the same chemical substance as a drug that was originally protected by chemical patents. Generic drugs are allowed for sale after the patents on the original drugs expire. Because the active chemical substance is the same, the medical profile of generics is equivalent in performance compared to their performance at the time when they were patented drugs. A generic drug has the same active pharmaceutical ingredient (API) as the original, but it may differ in some characteristics such as the manufacturing process, formulation, excipients, color, taste, and packaging. Although they may not be associated with a particular company, generic drugs are usually subject to government regulations in the countries in which they are dispensed. They are labeled with the name of the manufacturer and a generic non-proprietary name such as the United States Adopted Name (USAN) or International Nonproprietary Name (INN) of the drug. A generic ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Infliximab
Infliximab, a chimeric monoclonal antibody, sold under the brand name Remicade among others, is a medication used to treat a number of autoimmune diseases. This includes Crohn's disease, ulcerative colitis, rheumatoid arthritis, ankylosing spondylitis, psoriasis, psoriatic arthritis, and Behçet's disease. It is given by slow injection into a vein, typically at six- to eight-week intervals. Common side effects include infections, acute infusion reactions, and abdominal pain. Infliximab is a chimeric monoclonal antibody biologic. It seems to work by binding to and neutralizing TNF-α, preventing it from interacting with its receptors on the cell. TNF-α is a chemical messenger ( cytokine) and a key part of the autoimmune reaction. Infliximab was originally developed in mice as a mouse antibody. Because humans have immune reactions to mouse proteins, the mouse common domains were replaced with similar human antibody domains. They are monoclonal antibodies and hav ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Zarxio
Filgrastim, sold under the brand name Neupogen among others, is a medication used to treat low neutrophil count. Low neutrophil counts may occur with HIV/AIDS, following chemotherapy or radiation poisoning, or be of an unknown cause. It may also be used to increase white blood cells for gathering during leukapheresis. It is given either by injection into a vein or under the skin. Filgrastim is a leukocyte growth factor. Common side effects include fever, cough, chest pain, joint pain, vomiting, and hair loss. Severe side effects include splenic rupture and allergic reactions. It is unclear if use in pregnancy is safe for the baby. Filgrastim is a recombinant form of the naturally occurring granulocyte colony-stimulating factor (G-CSF). It works by stimulating the body to increase neutrophil production. Filgrastim was approved for medical use in the United States in 1991. It is on the World Health Organization's List of Essential Medicines. Filgrastim biosimilar medica ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Erythropoietin
Erythropoietin (; EPO), also known as erythropoetin, haematopoietin, or haemopoietin, is a glycoprotein cytokine secreted mainly by the kidneys in response to cellular hypoxia; it stimulates red blood cell production ( erythropoiesis) in the bone marrow. Low levels of EPO (around 10 mU/mL) are constantly secreted in sufficient quantities to compensate for normal red blood cell turnover. Common causes of cellular hypoxia resulting in elevated levels of EPO (up to 10 000 mU/mL) include any anemia, and hypoxemia due to chronic lung disease. Erythropoietin is largely synthesized by fibroblast-like type-1 interstitial cells, located primarily in the deep renal cortex in close association with the peritubular capillaries and proximal convoluted tubule; it is also produced in perisinusoidal cells in the liver. Liver production predominates in the fetal and perinatal period; renal production predominates in adulthood. It is homologous with thrombopoietin. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enoxaparin
Enoxaparin sodium, sold under the brand name Lovenox among others, is an anticoagulant medication (blood thinner). It is used to treat and prevent deep vein thrombosis (DVT) and pulmonary embolism (PE) including during pregnancy and following certain types of surgery. It is also used in those with acute coronary syndrome (ACS) and heart attacks. It is given by injection just under the skin or into a vein. It is also used during hemodialysis. Common side effects include bleeding, fever, and swelling of the legs. Bleeding may be serious especially in those who are undergoing a spinal tap. Use during pregnancy appears to be safe for the baby. Enoxaparin is in the low molecular weight heparin family of medications. Enoxaparin was first made in 1981 and approved for medical use in 1993. It is on the World Health Organization's List of Essential Medicines. Enoxaparin is sold under several brand names and is available as a generic medication. Enoxaparin is made from heparin. In ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
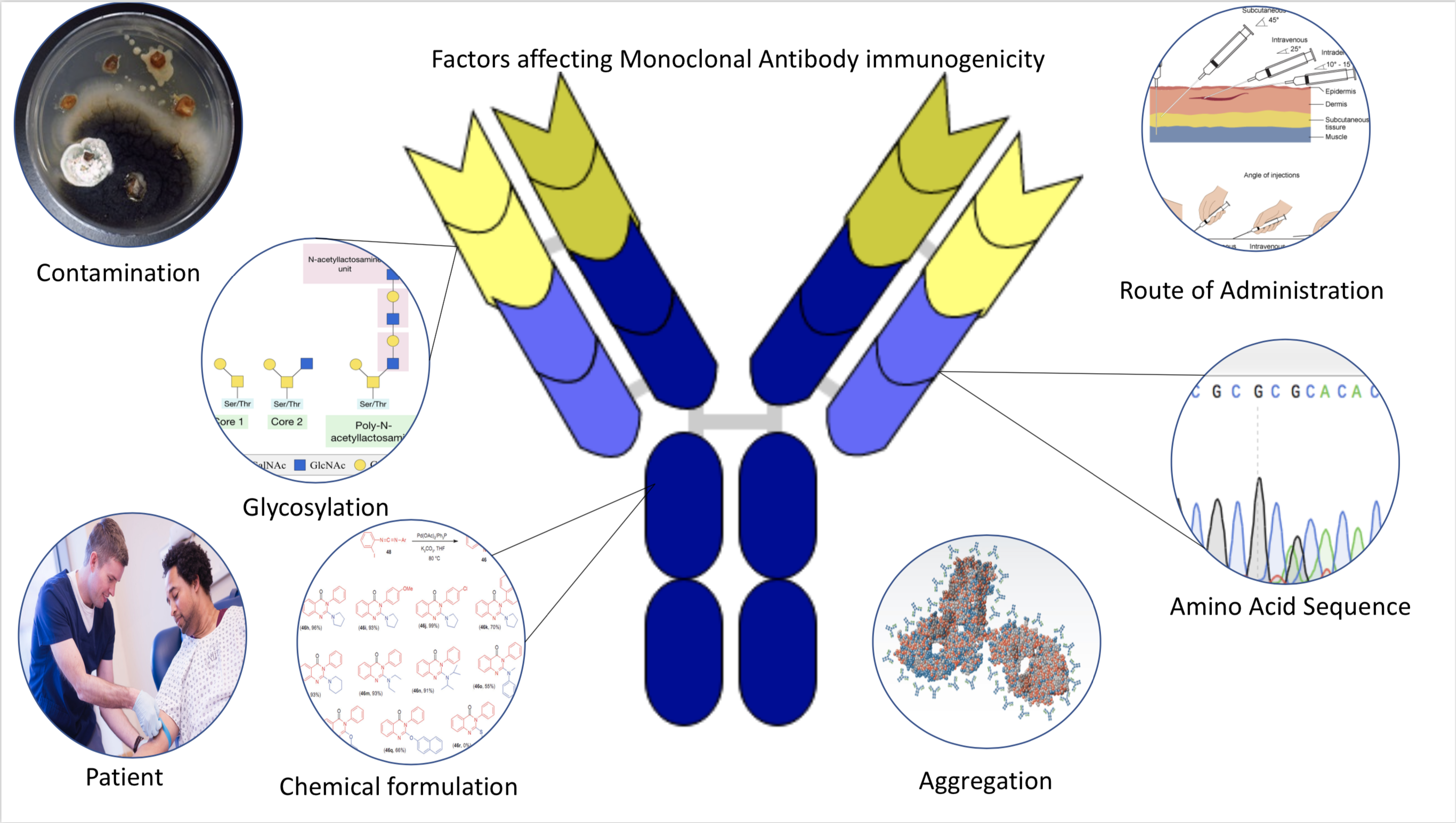
Immunogenicity
Immunogenicity is the ability of a foreign substance, such as an antigen, to provoke an immune response in the body of a human or other animal. It may be wanted or unwanted: * Wanted immunogenicity typically relates to vaccines, where the injection of an antigen (the vaccine) provokes an immune response against the pathogen, protecting the organism from future exposure. Immunogenicity is a central aspect of vaccine development. * Unwanted immunogenicity is an immune response by an organism against a therapeutic antigen. This reaction leads to production of anti-drug-antibodies (ADAs), inactivating the therapeutic effects of the treatment and potentially inducing adverse effects. A challenge in biotherapy is predicting the immunogenic potential of novel protein therapeutics. For example, immunogenicity data from high-income countries are not always transferable to low-income and middle-income countries. Another challenge is considering how the immunogenicity of vaccines changes wi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Sandoz
Sandoz Group AG is a Swiss company that focuses on generic pharmaceuticals and biosimilars. Prior to October 2023, it was part of a division of Novartis that was established in 2003, when Novartis united all of its generics businesses under the name Sandoz. Before this, the company existed as an independent pharmaceutical manufacturer until 1996, when it was merged with Ciba-Geigy to form Novartis. Prior to the merger, it specialized in medicines used in organ transplants, such as Sandimmune, and various antipsychotics and migraine medicines. Its headquarters were in Holzkirchen, Germany and after the spin-off from Novartis, the headquarters moved to Basel, Switzerland, with most of global functions operating from Holzkichen in Germany, Prague in Czechia, and Barcelona in Spain. Sandoz is the global leader in generic pharmaceuticals and biosimilars. History 1886–1995: Formation and initial growth The company was founded in 1886 by Alfred Kern (1850–1893) and Edouard S ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Recombinant DNA
Recombinant DNA (rDNA) molecules are DNA molecules formed by laboratory methods of genetic recombination (such as molecular cloning) that bring together genetic material from multiple sources, creating sequences that would not otherwise be found in the genome. Recombinant DNA is the general name for a piece of DNA that has been created by combining two or more fragments from different sources. Recombinant DNA is possible because DNA molecules from all organisms share the same chemical structure, differing only in the nucleotide sequence. Recombinant DNA molecules are sometimes called chimeric DNA because they can be made of material from two different species like the mythical chimera. rDNA technology uses palindromic sequences and leads to the production of sticky and blunt ends. The DNA sequences used in the construction of recombinant DNA molecules can originate from any species. For example, plant DNA can be joined to bacterial DNA, or human DNA can be joined with fun ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |