|
Anabasine
Anabasine is a pyridine and piperidine alkaloid found in the Tree Tobacco ('' Nicotiana glauca'') plant, a close relative of the common tobacco plant (''Nicotiana tabacum''). It is a structural isomer of, and chemically similar to, nicotine. Its principal (historical) industrial use is as an insecticide. Anabasine is present in trace amounts in tobacco smoke, and can be used as an indicator of a person's exposure to tobacco smoke. Pharmacology Anabasine is a nicotinic acetylcholine receptor agonist. In high doses, it produces a depolarizing block of nerve transmission, which can cause symptoms similar to those of nicotine poisoning and, ultimately, death by asystole. In larger amounts it is thought to be teratogenic in swine. The intravenous LD50 of anabasine ranges from 11 mg/kg to 16 mg/kg in mice, depending on the enantiomer In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enán ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nicotine
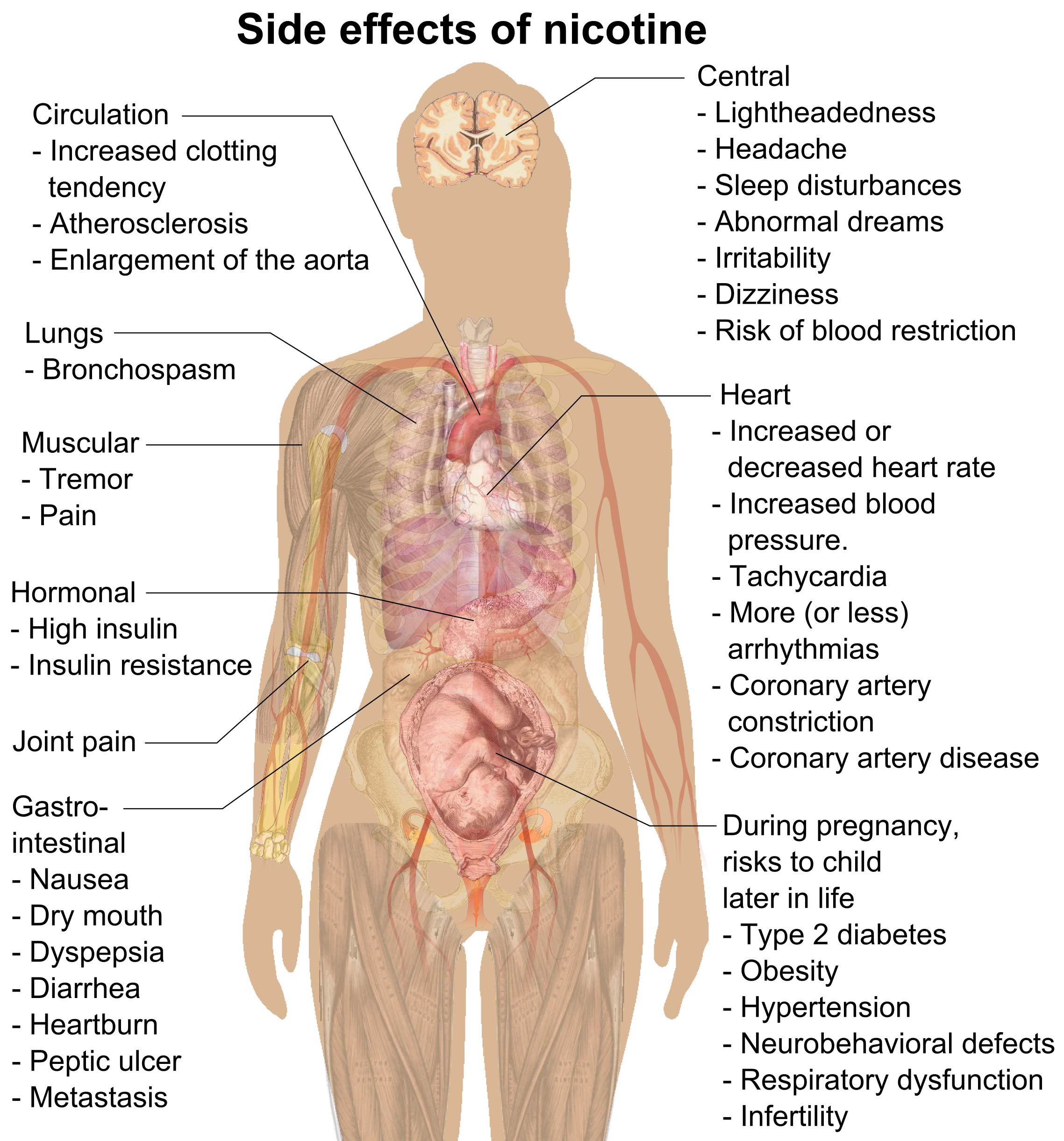
Nicotine is a naturally produced alkaloid in the nightshade family of plants (most predominantly in tobacco and '' Duboisia hopwoodii'') and is widely used recreationally as a stimulant and anxiolytic. As a pharmaceutical drug, it is used for smoking cessation to relieve withdrawal symptoms. Nicotine acts as a receptor agonist at most nicotinic acetylcholine receptors (nAChRs), except at two nicotinic receptor subunits ( nAChRα9 and nAChRα10) where it acts as a receptor antagonist. Nicotine constitutes approximately 0.6–3.0% of the dry weight of tobacco. Nicotine is also present at ppb-concentrations in edible plants in the family Solanaceae, including potatoes, tomatoes, and eggplants, though sources disagree on whether this has any biological significance to human consumers. It functions as an antiherbivore toxin; consequently, nicotine was widely used as an insecticide in the past, and neonicotinoids (structurally similar to nicotine), such as imidac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Insecticide
Insecticides are substances used to kill insects. They include ovicides and larvicides used against insect eggs and larvae, respectively. Insecticides are used in agriculture, medicine, industry and by consumers. Insecticides are claimed to be a major factor behind the increase in the 20th-century's agricultural productivity. Nearly all insecticides have the potential to significantly alter ecosystems; many are toxic to humans and/or animals; some become concentrated as they spread along the food chain. Insecticides can be classified into two major groups: systemic insecticides, which have residual or long term activity; and contact insecticides, which have no residual activity. The mode of action describes how the pesticide kills or inactivates a pest. It provides another way of classifying insecticides. Mode of action can be important in understanding whether an insecticide will be toxic to unrelated species, such as fish, birds and mammals. Insecticides may be repellen ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyridine Alkaloids
Pyridine alkaloids are a class of alkaloids, nitrogen-containing chemical compounds widely found in plants, that contain a pyridine ring. Examples include nicotine and anabasine which are found in plants of the genus ''Nicotiana'' including tobacco. Alkaloids with a pyridine partial structure are usually further subdivided according to their occurrence and their biogenetic origin. The most important examples of pyridine alkaloids are the nicotine and anabasine, which are found in tobacco, the areca alkaloids in betel and ricinine in castor oil. File:Nikotin - Nicotine.svg, nicotine File:Anabasine.svg, anabasine File:Ricinine.png, ricinine Ricinine is a toxic alkaloid found in the castor plant. It can serve as a biomarker of ricin poisoning. It was first isolated from the castor seeds by Tuson in 1864. Ricinine has insecticidal effects. It sublimes between 170 and 180 °C at 20 m ... References External links * {{alkaloid-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
TC-1698
TC-1698 is a drug developed by Targacept which acts as a partial agonist for the α7 subtype of neural nicotinic acetylcholine receptors. It has neuroprotective effects in animal studies, and has been used as a lead compound to find further potent derivatives. See also * Anabasine Anabasine is a pyridine and piperidine alkaloid found in the Tree Tobacco ('' Nicotiana glauca'') plant, a close relative of the common tobacco plant (''Nicotiana tabacum''). It is a structural isomer of, and chemically similar to, nicotine. Its ... References Nicotinic agonists Stimulants 3-Pyridyl compounds {{nervous-system-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyridine
Pyridine is a basic heterocyclic organic compound with the chemical formula . It is structurally related to benzene, with one methine group replaced by a nitrogen atom. It is a highly flammable, weakly alkaline, water-miscible liquid with a distinctive, unpleasant fish-like smell. Pyridine is colorless, but older or impure samples can appear yellow, due to the formation of extended, unsaturated polymeric chains, which show significant electrical conductivity. The pyridine ring occurs in many important compounds, including agrochemicals, pharmaceuticals, and vitamins. Historically, pyridine was produced from coal tar. As of 2016, it is synthesized on the scale of about 20,000 tons per year worldwide. Properties Physical properties The molecular electric dipole moment is 2.2 debyes. Pyridine is diamagnetic and has a diamagnetic susceptibility of −48.7 × 10−6 cm3·mol−1. The standard enthalpy of formation is 100.2 kJ·mol−1 in the l ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Enantiomer
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical antipode – is one of two stereoisomers that are non-superposable onto their own mirror image. Enantiomers are much like one's right and left hands, when looking at the same face, they cannot be superposed onto each other. No amount of reorientation will allow the four unique groups on the chiral carbon (see Chirality (chemistry)) to line up exactly. The number of stereoisomers a molecule has can be determined by the number of chiral carbons it has. Stereoisomers include both enantiomers and diastereomers. Diastereomers, like enantiomers, share the same molecular formula and are non-superposable onto each other however, they are not mirror images of each other. A molecule with chirality rotates plane-polarized light. A mixture of equals am ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Piperidine Alkaloids
Piperidine alkaloids are naturally occurring chemical compounds from the group of alkaloids, which are chemically derived from piperidine. Alkaloids with a piperidine building block are widespread and are usually further subdivided according to their occurrence and biogenetic origin. The most important representative of piperidine alkaloids is piperine, which is responsible for the pungent taste of pepper. The piperidine alkaloids also include the sedum alkaloids (e.g. sedamine), pelletierine, the lobelia alkaloids (e.g. lobeline), the conium alkaloids (such as coniine) and the pinus alkaloids. Piperin.svg, Piperine Lobeline Structural Formula V2.svg, Lobeline (S)-Coniine Structural Formula V.1.svg, (''S'')-Coniine (2R,8R)-Sedamine Structural Formula V4.svg, Sedamin Solenopsin Structural Formula V.1.svg, Solenopsin Solenopsin is a lipophilic alkaloid with the molecular formula C17H35N found in the venom of fire ants (''Solenopsis''). It is considered the primary toxin in ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Plant Toxin Insecticides
Plants are predominantly photosynthetic eukaryotes of the kingdom Plantae. Historically, the plant kingdom encompassed all living things that were not animals, and included algae and fungi; however, all current definitions of Plantae exclude the fungi and some algae, as well as the prokaryotes (the archaea and bacteria). By one definition, plants form the clade Viridiplantae (Latin name for "green plants") which is sister of the Glaucophyta, and consists of the green algae and Embryophyta (land plants). The latter includes the flowering plants, conifers and other gymnosperms, ferns and their allies, hornworts, liverworts, and mosses. Most plants are multicellular organisms. Green plants obtain most of their energy from sunlight via photosynthesis by primary chloroplasts that are derived from endosymbiosis with cyanobacteria. Their chloroplasts contain chlorophylls a and b, which gives them their green color. Some plants are parasitic or mycotrophic and have lost the ability ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alkaloids Found In Nicotiana
Alkaloids are a class of base (chemistry), basic, natural product, naturally occurring organic compounds that contain at least one nitrogen atom. This group also includes some related compounds with neutral and even weakly acidic properties. Some synthetic compounds of similar structure may also be termed alkaloids. In addition to carbon, hydrogen and nitrogen, alkaloids may also contain oxygen, sulfur and, more rarely, other elements such as chlorine, bromine, and phosphorus.Chemical Encyclopedia: alkaloids xumuk.ru Alkaloids are produced by a large variety of organisms including bacteria, fungus, fungi, Medicinal plant, plants, and animals. They can be purified from crude extracts of these organisms by acid-base extraction, or solvent extractions followed by silica-gel column chromatography. [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nicotinic Agonists
A nicotinic agonist is a drug that mimics the action of acetylcholine (ACh) at nicotinic acetylcholine receptors (nAChRs). The nAChR is named for its affinity for nicotine. Examples include nicotine (by definition), acetylcholine (the endogenous agonist of nAChRs), choline, epibatidine, lobeline, varenicline and cytisine. History Nicotine has been known for centuries for its intoxicating effect. It was first isolated in 1828 from the tobacco plant by German chemists Posselt and Reimann. The discovery of positive effects from nicotine on animal memory was discovered by in vivo researches in the mid 1980s. Those researches led to a new era in studies of nicotinic acetylcholine receptor (nAChR) and their stimulation but until then the focus had mainly been on nicotine addiction. The development of nAChR agonists began in the early 1990s after the discovery of nicotine's positive effects. Some research showed a possible therapy option in preclinical researches. ABT-418 was on ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Anatabine
Anatabine (uh-nat-uh-been,-bin) is one of the minor alkaloids found in plants in the family Solanaceae, which includes the tobacco plant and tomato. Commercial tobacco plants typically produce alkaloids at levels between 2% and 4% of total dry weight, with nicotine accounting for about 90% of the total alkaloid content, and the related compounds anatabine, nornicotine, and anabasine making up nearly all the rest. These compounds are thought to be biologically active, and part of plants' natural defense system against insects. Anatabine has anti-inflammatory activity partly through inhibition of STAT3 phosphorylation in vitro and in vivo. Pharmacology On a biochemical level, it appears to be active against certain nicotinic acetylcholine receptors. Commercial development Star Scientific developed and sold the compound as a dietary supplement primarily through GNC up until mid 2014. Subsequently, Rock Creek Pharmaceuticals (formerly a subsidiary of Star Scientific), headquar ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Bicyclic
In chemistry, a bicyclic molecule () is a molecule that features two joined rings. Bicyclic structures occur widely, for example in many biologically important molecules like α-thujene and camphor. A bicyclic compound can be carbocyclic (all of the ring atoms are carbons), or heterocyclic (the rings' atoms consist of at least two elements), like DABCO. Moreover, the two rings can both be aliphatic (''e.g.'' decalin and norbornane), or can be aromatic (''e.g.'' naphthalene), or a combination of aliphatic and aromatic (''e.g.'' tetralin). Three modes of ring junction are possible for a bicyclic compound: * In spirocyclic compounds, the two rings share only one single atom, the spiro atom, which is usually a quaternary carbon. An example of a spirocyclic compound is the photochromic switch spiropyran. * In fused/condensed bicyclic compounds, two rings share two adjacent atoms. In other words, the rings share one covalent bond, ''i.e.'' the so-called bridgehead atoms are di ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |