|
Alpha-keratin
Alpha-keratin, or α-keratin, is a type of keratin found in vertebrates. This protein is the primary component in hairs, horns, mammalian claws, nails and the epidermis layer of the skin. α-keratin is a fibrous structural protein, meaning it is made up of amino acids that form a repeating secondary structure. The secondary structure of α-keratin is very similar to that of a traditional protein α-helix and forms a coiled coil. Due to its tightly wound structure, it can function as one of the strongest biological materials and has various functions in mammals, from predatory claws to hair for warmth. α-keratin is synthesized through protein biosynthesis, utilizing transcription and translation, but as the cell matures and is full of α-keratin, it dies, creating a strong non-vascular unit of keratinized tissue. Structure α-keratin is a polypeptide chain, typically high in alanine, leucine, arginine, and cysteine, that forms a right-handed α-helix. Two of these polypepti ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hair
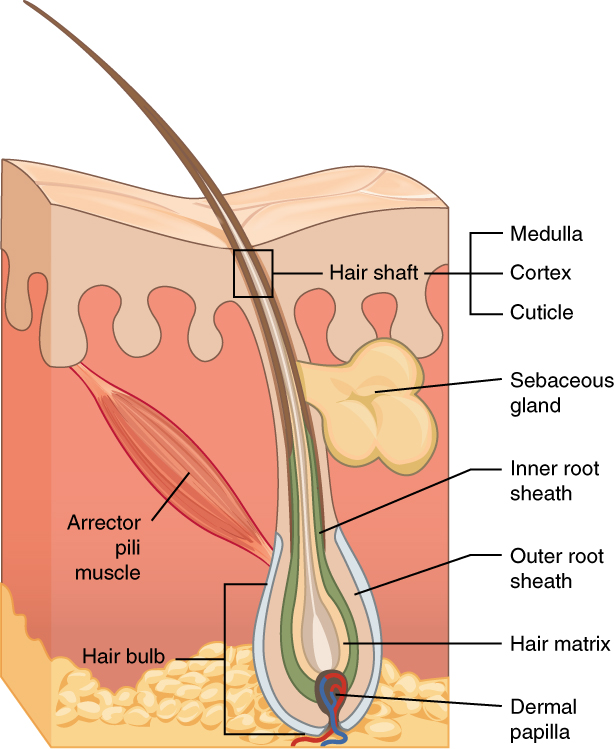
Hair is a protein filament that grows from follicles found in the dermis. Hair is one of the defining characteristics of mammals. The human body, apart from areas of glabrous skin, is covered in follicles which produce thick terminal and fine vellus hair. Most common interest in hair is focused on hair growth, hair types, and hair care, but hair is also an important biomaterial primarily composed of protein, notably alpha-keratin. Attitudes towards different forms of hair, such as hairstyles and hair removal, vary widely across different cultures and historical periods, but it is often used to indicate a person's personal beliefs or social position, such as their age, sex, or religion. Overview The word "hair" usually refers to two distinct structures: #the part beneath the skin, called the hair follicle, or, when pulled from the skin, the bulb or root. This organ is located in the dermis and maintains stem cells, which not only re-grow the hair after it falls out, bu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Coiled Coil
A coiled coil is a structural motif in proteins in which 2–7 alpha-helices are coiled together like the strands of a rope. (Dimers and trimers are the most common types.) Many coiled coil-type proteins are involved in important biological functions, such as the regulation of gene expression — e.g., transcription factors. Notable examples are the oncoproteins c-Fos and c-Jun, as well as the muscle protein tropomyosin. Discovery The possibility of coiled coils for α-keratin was initially somewhat controversial. Linus Pauling and Francis Crick independently came to the conclusion that this was possible at about the same time. In the summer of 1952, Pauling visited the laboratory in England where Crick worked. Pauling and Crick met and spoke about various topics; at one point, Crick asked whether Pauling had considered "coiled coils" (Crick came up with the term), to which Pauling said he had. Upon returning to the United States, Pauling resumed research on the topic. He con ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nail (anatomy)
A nail is a claw-like plate found at the tip of the fingers and toes on most primates. Nails correspond to the claws found in other animals. Fingernails and toenails are made of a tough protective protein called alpha-keratin, which is a polymer. Alpha-keratin is found in the hooves, claws, and horns of vertebrates. Structure The nail consists of the nail plate, the nail matrix and the nail bed below it, and the grooves surrounding it. Parts of the nail The matrix, sometimes called the ''matrix unguis'', keratogenous membrane, nail matrix, or onychostroma, is the active tissue (or germinal matrix) that generates cells, which harden as they move outward from the nail root to the nail plate. It is the part of the nail bed that is beneath the nail and contains nerves, lymph and blood vessels. The matrix produces cells that become the nail plate. The width and thickness of the nail plate is determined by the size, length, and thickness of the matrix, while the shape of the fing ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Keratin
Keratin () is one of a family of structural fibrous proteins also known as ''scleroproteins''. Alpha-keratin (α-keratin) is a type of keratin found in vertebrates. It is the key structural material making up Scale (anatomy), scales, hair, Nail (anatomy), nails, feathers, horn (anatomy), horns, claws, Hoof, hooves, and the outer layer of skin among vertebrates. Keratin also protects epithelial cells from damage or stress. Keratin is extremely insoluble in water and organic solvents. Keratin monomers assemble into bundles to form intermediate filaments, which are tough and form strong mineralization (biology), unmineralized epidermal appendages found in reptiles, birds, amphibians, and mammals. Excessive keratinization participate in fortification of certain tissues such as in horns of cattle and rhinos, and armadillos' osteoderm. The only other biology, biological matter known to approximate the toughness of keratinized tissue is chitin. Keratin comes in two types, the primitive, ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arginine
Arginine is the amino acid with the formula (H2N)(HN)CN(H)(CH2)3CH(NH2)CO2H. The molecule features a guanidino group appended to a standard amino acid framework. At physiological pH, the carboxylic acid is deprotonated (−CO2−) and both the amino and guanidino groups are protonated, resulting in a cation. Only the -arginine (symbol Arg or R) enantiomer is found naturally. Arg residues are common components of proteins. It is encoded by the codons CGU, CGC, CGA, CGG, AGA, and AGG. The guanidine group in arginine is the precursor for the biosynthesis of nitric oxide. Like all amino acids, it is a white, water-soluble solid. History Arginine was first isolated in 1886 from yellow lupin seedlings by the German chemist Ernst Schulze and his assistant Ernst Steiger. He named it from the Greek ''árgyros'' (ἄργυρος) meaning "silver" due to the silver-white appearance of arginine nitrate crystals. In 1897, Schulze and Ernst Winterstein (1865–1949) determined the structu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disulfide Bonds
In biochemistry, a disulfide (or disulphide in British English) refers to a functional group with the structure . The linkage is also called an SS-bond or sometimes a disulfide bridge and is usually derived by the coupling of two thiol groups. In biology, disulfide bridges formed between thiol groups in two cysteine residues are an important component of the secondary and tertiary structure of proteins. '' Persulfide'' usually refers to compounds. In inorganic chemistry disulfide usually refers to the corresponding anion (−S−S−). Organic disulfides Symmetrical disulfides are compounds of the formula . Most disulfides encountered in organo sulfur chemistry are symmetrical disulfides. Unsymmetrical disulfides (also called heterodisulfides) are compounds of the formula . They are less common in organic chemistry, but most disulfides in nature are unsymmetrical. Properties The disulfide bonds are strong, with a typical bond dissociation energy of 60 kcal/mol (251&nb ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Peptide
Peptides (, ) are short chains of amino acids linked by peptide bonds. Long chains of amino acids are called proteins. Chains of fewer than twenty amino acids are called oligopeptides, and include dipeptides, tripeptides, and tetrapeptides. A polypeptide is a longer, continuous, unbranched peptide chain. Hence, peptides fall under the broad chemical classes of biological polymers and oligomers, alongside nucleic acids, oligosaccharides, polysaccharides, and others. A polypeptide that contains more than approximately 50 amino acids is known as a protein. Proteins consist of one or more polypeptides arranged in a biologically functional way, often bound to ligands such as coenzymes and cofactors, or to another protein or other macromolecule such as DNA or RNA, or to complex macromolecular assemblies. Amino acids that have been incorporated into peptides are termed residues. A water molecule is released during formation of each amide bond.. All peptides except cyc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Alanine
Alanine (symbol Ala or A), or α-alanine, is an α-amino acid that is used in the biosynthesis of proteins. It contains an amine group and a carboxylic acid group, both attached to the central carbon atom which also carries a methyl group side chain. Consequently, its IUPAC systematic name is 2-aminopropanoic acid, and it is classified as a nonpolar, aliphatic α-amino acid. Under biological conditions, it exists in its zwitterionic form with its amine group protonated (as −NH3+) and its carboxyl group deprotonated (as −CO2−). It is non-essential to humans as it can be synthesised metabolically and does not need to be present in the diet. It is encoded by all codons starting with GC (GCU, GCC, GCA, and GCG). The L-isomer of alanine (left-handed) is the one that is incorporated into proteins. L-alanine is second only to leucine in rate of occurrence, accounting for 7.8% of the primary structure in a sample of 1,150 proteins. The right-handed form, D-alanine, occurs ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leucine
Leucine (symbol Leu or L) is an essential amino acid that is used in the biosynthesis of proteins. Leucine is an α-amino acid, meaning it contains an α- amino group (which is in the protonated −NH3+ form under biological conditions), an α- carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain isobutyl group, making it a non-polar aliphatic amino acid. It is essential in humans, meaning the body cannot synthesize it: it must be obtained from the diet. Human dietary sources are foods that contain protein, such as meats, dairy products, soy products, and beans and other legumes. It is encoded by the codons UUA, UUG, CUU, CUC, CUA, and CUG. Like valine and isoleucine, leucine is a branched-chain amino acid. The primary metabolic end products of leucine metabolism are acetyl-CoA and acetoacetate; consequently, it is one of the two exclusively ketogenic amino acids, with lysine being the other. It is the most import ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimer (chemistry)
A dimer () ('' di-'', "two" + ''-mer'', "parts") is an oligomer consisting of two monomers joined by bonds that can be either strong or weak, covalent or intermolecular. Dimers also have significant implications in polymer chemistry, inorganic chemistry, and biochemistry. The term ''homodimer'' is used when the two molecules are identical (e.g. A–A) and ''heterodimer'' when they are not (e.g. A–B). The reverse of dimerization is often called dissociation. When two oppositely charged ions associate into dimers, they are referred to as ''Bjerrum pairs'', after Niels Bjerrum. Noncovalent dimers Anhydrous carboxylic acids form dimers by hydrogen bonding of the acidic hydrogen and the carbonyl oxygen. For example, acetic acid forms a dimer in the gas phase, where the monomer units are held together by hydrogen bonds. Under special conditions, most OH-containing molecules form dimers, e.g. the water dimer. Excimers and exciplexes are excited structures with a short lif ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cysteine
Cysteine (symbol Cys or C; ) is a semiessential proteinogenic amino acid with the formula . The thiol side chain in cysteine often participates in enzymatic reactions as a nucleophile. When present as a deprotonated catalytic residue, sometimes the symbol Cyz is used. The deprotonated form can generally be described by the symbol Cym as well. The thiol is susceptible to oxidation to give the disulfide derivative cystine, which serves an important structural role in many proteins. In this case, the symbol Cyx is sometimes used. When used as a food additive, it has the E number E920. Cysteine is encoded by the codons UGU and UGC. The sulfur-containing amino acids cysteine and methionine are more easily oxidized than the other amino acids. Structure Like other amino acids (not as a residue of a protein), cysteine exists as a zwitterion. Cysteine has chirality in the older / notation based on homology to - and -glyceraldehyde. In the newer ''R''/''S'' system of design ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Vascular
The blood vessels are the components of the circulatory system that transport blood throughout the human body. These vessels transport blood cells, nutrients, and oxygen to the tissues of the body. They also take waste and carbon dioxide away from the tissues. Blood vessels are needed to sustain life, because all of the body's tissues rely on their functionality. There are five types of blood vessels: the arteries, which carry the blood away from the heart; the arterioles; the capillaries, where the exchange of water and chemicals between the blood and the tissues occurs; the venules; and the veins, which carry blood from the capillaries back towards the heart. The word ''vascular'', meaning relating to the blood vessels, is derived from the Latin ''vas'', meaning vessel. Some structures – such as cartilage, the epithelium, and the lens and cornea of the eye – do not contain blood vessels and are labeled ''avascular''. Etymology * artery: late Middle English; from Lat ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |