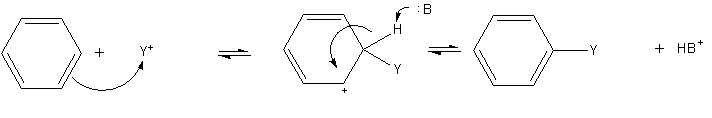
|
Aromatics
Aromatic compounds or arenes are organic compounds "with a chemistry typified by benzene" and "cyclically conjugated." The word "aromatic" originates from the past grouping of molecules based on odor, before their general chemical properties were understood. The current definition of aromatic compounds does not have any relation to their odor. Aromatic compounds are now defined as cyclic compounds satisfying Hückel's rule. Aromatic compounds have the following general properties: * Typically unreactive * Often non polar and hydrophobic * High carbon-hydrogen ratio * Burn with a strong sooty yellow flame, due to high C:H ratio * Undergo electrophilic substitution reactions and nucleophilic aromatic substitutions Arenes are typically split into two categories - benzoids, that contain a benzene derivative and follow the benzene ring model, and non-benzoids that contain other aromatic cyclic derivatives. Aromatic compounds are commonly used in organic synthesis and are involved in m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzene Structural Diagram
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of petroleum and is one of the elementary petrochemicals. Due to the cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon. Benzene is a colorless and highly Combustibility and flammability, flammable liquid with a sweet smell, and is partially responsible for the aroma of gasoline. It is used primarily as a Precursor (chemistry), precursor to the manufacture of chemicals with more complex structures, such as ethylbenzene and cumene, of which billions of kilograms are produced annually. Although benzene is a major Chemical industry, industrial che ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzene Orbitals
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of petroleum and is one of the elementary petrochemicals. Due to the cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon. Benzene is a colorless and highly flammable liquid with a sweet smell, and is partially responsible for the aroma of gasoline. It is used primarily as a precursor to the manufacture of chemicals with more complex structures, such as ethylbenzene and cumene, of which billions of kilograms are produced annually. Although benzene is a major industrial chemical, it finds limited use in consumer items because of its toxicity. Benzene is a volatile organic compound. Benzene is classified as a c ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Benzene With Hydrogens
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen atoms, benzene is classed as a hydrocarbon. Benzene is a natural constituent of petroleum and is one of the elementary petrochemicals. Due to the cyclic continuous pi bonds between the carbon atoms, benzene is classed as an aromatic hydrocarbon. Benzene is a colorless and highly flammable liquid with a sweet smell, and is partially responsible for the aroma of gasoline. It is used primarily as a precursor to the manufacture of chemicals with more complex structures, such as ethylbenzene and cumene, of which billions of kilograms are produced annually. Although benzene is a major industrial chemical, it finds limited use in consumer items because of its toxicity. Benzene is a volatile organic compound. Benzene is classified as a carci ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Toluene
Toluene (), also known as toluol (), is a substituted aromatic hydrocarbon with the chemical formula , often abbreviated as , where Ph stands for the phenyl group. It is a colorless, water Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known liv ...-insoluble liquid with the odor associated with paint thinners. It is a mono-substituted benzene derivative, consisting of a methyl group (CH3) attached to a phenyl group by a single bond. As such, its systematic IUPAC nomenclature of organic chemistry, IUPAC name is methylbenzene. Toluene is predominantly used as an industrial feedstock and a solvent. As the solvent in some types of paint thinner, permanent markers, contact cement and certain types of glue, toluene is sometimes used as a recreational inhalant and has the potential of causin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hückel's Rule
In organic chemistry, Hückel's rule predicts that a planar ring molecule will have aromatic properties if it has 4''n'' + 2 π-electrons, where ''n'' is a non-negative integer. The quantum mechanical basis for its formulation was first worked out by physical chemist Erich Hückel in 1931. The succinct expression as the 4''n'' + 2 rule has been attributed to W. v. E. Doering (1951), although several authors were using this form at around the same time. In agreement with the Möbius–Hückel concept, a cyclic ring molecule follows Hückel's rule when the number of its π-electrons equals 4''n'' + 2, although clearcut examples are really only established for values of ''n'' = 0 up to about ''n'' = 6. Hückel's rule was originally based on calculations using the Hückel method, although it can also be justified by considering a particle in a ring system, by the LCAO method and by the Pariser–Parr–Pople method. Aroma ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Xylene
In organic chemistry, xylene or xylol (; IUPAC name: dimethylbenzene) are any of three organic compounds with the formula . They are derived from the substitution of two hydrogen atoms with methyl groups in a benzene ring; which hydrogens are substituted determines which of three structural isomers results. It is a colorless, flammable, slightly greasy liquid of great industrial value. The mixture is referred to as both xylene and, more precisely, xylenes. Mixed xylenes refers to a mixture of the xylenes plus ethylbenzene. The four compounds have identical molecular formulas . Typically the four compounds are produced together by various catalytic reforming and pyrolysis methods. Occurrence and production Xylenes are an important petrochemical produced by catalytic reforming and also by coal carbonisation in the manufacture of coke fuel. They also occur in crude oil in concentrations of about 0.5–1%, depending on the source. Small quantities occur in gasoline and aircra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ortho Meta Para
Ortho- is a Greek prefix meaning “straight”, “upright”, “right” or “correct”. Ortho may refer to: * Ortho, Belgium, a village in the Belgian province of Luxembourg Science * List of commonly used taxonomic affixes (ortho-) * Arene substitution patterns, two substituents that occupy adjacent positions on an aromatic ring * Chlordane, an organochlorine compound that was used as a pesticide Mathematics * Orthogonal, a synonym for perpendicular * Orthonormal, the property that a collection of vectors are mutually perpendicular and each of unit magnitude * Orthodrome, a synonym for great circle, a geodesic on the sphere * Orthographic projection, a parallel projection onto a perpendicular plane Medicine * Orthomyxovirus, a family of viruses to which influenza belongs * Orthodontics, a specialty of dentistry concerned with the study and treatment of malocclusions * Orthopedic, the study of the musculoskeletal system * Ortho-DOT, a psychedelic drug * Ortho-cept and ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Three-center Two-electron Bond
A three-center two-electron (3c–2e) bond is an electron-deficient chemical bond where three atoms share two electrons. The combination of three atomic orbitals form three molecular orbitals: one bonding, one ''non''-bonding, and one ''anti''-bonding. The two electrons go into the bonding orbital, resulting in a net bonding effect and constituting a chemical bond among all three atoms. In many common bonds of this type, the bonding orbital is shifted towards two of the three atoms instead of being spread equally among all three. Example molecules with 3c–2e bonds are the trihydrogen cation () and diborane (). In these two structures, the three atoms in each 3c–2e bond form an angular geometry, leading to a bent bond. Boranes and carboranes An extended version of the 3c–2e bond model features heavily in cluster compounds described by the polyhedral skeletal electron pair theory, such as boranes and carboranes. These molecules derive their stability from having a com ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Robert Robinson (organic Chemist)
Sir Robert Robinson (13 September 1886 – 8 February 1975) was a British organic chemist and List of Nobel laureates, Nobel laureate recognised in 1947 for his research on plant dyestuffs (anthocyanins) and alkaloids. In 1947, he also received the Medal of Freedom (1945), Medal of Freedom with Silver Palm. Biography Early life He was born at Rufford House Farm, near Chesterfield, Derbyshire, Chesterfield, Derbyshire the son of James Bradbury Robinson, a maker of surgical dressings, and his wife, Jane Davenport. Robinson went to school at the Brookfield Community School, Chesterfield, Chesterfield Grammar School and the private Fulneck School. He then studied chemistry at the Victoria University of Manchester, University of Manchester, graduating BSc in 1905. In 1907 he was awarded an 1851 Research Fellowship from the Royal Commission for the Exhibition of 1851 to continue his research at the University of Manchester. He was appointed as the first Professor of Pure and App ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Resonance (chemistry)
In chemistry, resonance, also called mesomerism, is a way of describing Chemical bond, bonding in certain molecules or polyatomic ions by the combination of several contributing structures (or ''forms'', also variously known as ''resonance structures'' or ''canonical structures'') into a resonance hybrid (or ''hybrid structure'') in valence bond theory. It has particular value for analyzing delocalized electrons where the bonding cannot be expressed by one single Lewis structure. The resonance hybrid is the accurate structure for a molecule or ion; it is an average of the theoretical (or hypothetical) contributing structures. Overview Under the framework of valence bond theory, resonance is an extension of the idea that the bonding in a chemical species can be described by a Lewis structure. For many chemical species, a single Lewis structure, consisting of atoms obeying the octet rule, possibly bearing formal charges, and connected by bonds of positive integer order, is suffi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |