|
Antimycin Congeners
Antimycins are produced as secondary metabolites by ''Streptomyces'' bacteria, a soil bacteria. These specialized metabolites likely function to kill neighboring organisms in order to provide the streptomyces bacteria with a competitive edge. Chemical structures Biosynthesis Antimycins are produced by a non-ribosomal peptide synthetase (NRPS)/polyketide synthase (PKS) assembly complex which acts as an assembly line for antimycin production. The assembly is genetically coded for by the ant gene family. The assembly requires 14 proteins, AntBCDEFGHIJKLMNO, which shuttle the intermediates along the assembly line through a series of transesterifications, Carbonyl reduction, keto reductions, thiolations (addition of a sulfur containing group), Condensation reaction, condensations, anadenylations The last two steps involving AntB and AntO are tailoring steps. The following steps describe chemically what the Ant Enzymes do in order to synthesize Antimycin. Synthesis begins wit ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Streptomyces
''Streptomyces'', from στρεπτός (''streptós''), meaning "twisted", and μύκης (''múkés''), meaning "fungus", is the largest genus of Actinomycetota, and the type genus of the family Streptomycetaceae. Over 700 species of ''Streptomyces'' bacteria have been described. As with the other Actinomycetota, streptomycetes are gram-positive, and have very large genomes with high GC content. Found predominantly in soil and decaying vegetation, most streptomycetes produce spores, and are noted for their distinct "earthy" odor that results from production of a volatile metabolite, geosmin. Different strains of the same species may colonize very diverse environments. Streptomycetes are characterised by a complex secondary metabolism. Between 5-23% (average: 12%) of the protein-coding genes of each ''Streptomyces'' species are implicated in secondary metabolism. Streptomycetes produce over two-thirds of the clinically useful antibiotics of natural origin (e.g., neomy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acyl-CoA Synthetase
Acyl-CoA synthetases, also known as acyl-CoA ligases, are enzymes that "activate" fatty acids by thioesterification to coenzyme A. It represents the initial step of fatty acid metabolism so that fatty acids can participate in catabolic and anabolic pathways. Among these are, for example, the synthesis of triacylglycerol, phospholipids, plasmalogens, sphingolipids, the degradation of fatty acids for energy production, the conversion to alcohols or aldehydes, the elongation of fatty acids, the insertion and removal of double bonds or the covalent binding to proteins. The members of this family mainly activate fatty acids, but there are also members that activate other substrates instead, such as AACS, which activates the keto acid acetoacetic acid, or ACSF3, which activates the dicarboxylic acids methylmalonic acid and malonic acid. Reaction Acyl-CoA synthetases catalyze fatty acid activation, which consists of 2 steps. First, an ATP-dependent adenylation and release of pyro ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Antimycin A
Antimycin A (more exactly antimycin A1b) is a secondary metabolite produced by '' Streptomyces'' bacteria and a member of a group of related compounds called antimycins. Antimycin A is classified as an extremely hazardous substance in the United States, as defined in Section 302 of the U.S. Emergency Planning and Community Right-to-Know Act (42 U.S.C. 11002), and is subject to strict reporting requirements by facilities which produce, store, or use it in significant quantities. Use Antimycin A is the active ingredient in Fintrol, a chemical piscicide (fish poison) used in fisheries management. Antimycin A was first discovered in 1945 and registered for use as a fish toxicant in 1960. Fintrol is the only currently registered product containing antimycin A and is classified as a restricted use pesticide because of its aquatic toxicity and requirement for highly specialized training in order to use it. In 1993, several toxicology studies were submitted to the United States ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Acyltransferase
Acyltransferase is a type of transferase enzyme that acts upon acyl In chemistry, an acyl group is a moiety derived by the removal of one or more hydroxyl groups from an oxoacid, including inorganic acids. It contains a double-bonded oxygen atom and an organyl group () or hydrogen in the case of formyl grou ... groups. Examples include: * Glycerol-3-phosphate acyltransferases * Glyceronephosphate O-acyltransferase * Lecithin-cholesterol acyltransferase * Long-chain-alcohol O-fatty-acyltransferase See also * Acetyltransferase External links * Transferases EC 2.3 {{2.3-enzyme-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Lactone
Lactones are cyclic carboxylic esters. They are derived from the corresponding hydroxycarboxylic acids by esterification. They can be saturated or unsaturated. Lactones are formed by lactonization, the intramolecular esterification of the corresponding hydroxycarboxylic acids. Nomenclature Greek alphabet#Letters, Greek prefixes in alphabetical order indicate ring size. Lactones are usually named according to the precursor acid molecule (''aceto'' = 2 carbon atoms, ''propio'' = 3, ''butyro'' = 4, ''valero'' = 5, ''capro'' = 6, etc.), with a ''-lactone'' suffix and a Greek letter prefix that specifies the number of carbon atoms in the heterocycle — that is, the distance between the relevant -OH and the -COOH groups along said backbone. The first carbon atom after the carbon in the -COOH group on the parent compound is labelled α, the second will be labeled β, and so forth. Therefore, the prefixes also indicate the size of the lactone ring: α-lactone = 3-membered ring, β-lac ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Protein Domain
In molecular biology, a protein domain is a region of a protein's Peptide, polypeptide chain that is self-stabilizing and that Protein folding, folds independently from the rest. Each domain forms a compact folded Protein tertiary structure, three-dimensional structure. Many proteins consist of several domains, and a domain may appear in a variety of different proteins. Molecular evolution uses domains as building blocks and these may be recombined in different arrangements to create proteins with different functions. In general, domains vary in length from between about 50 amino acids up to 250 amino acids in length. The shortest domains, such as zinc fingers, are stabilized by metal ions or Disulfide bond, disulfide bridges. Domains often form functional units, such as the calcium-binding EF-hand, EF hand domain of calmodulin. Because they are independently stable, domains can be "swapped" by genetic engineering between one protein and another to make chimera (protein), chimeric ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
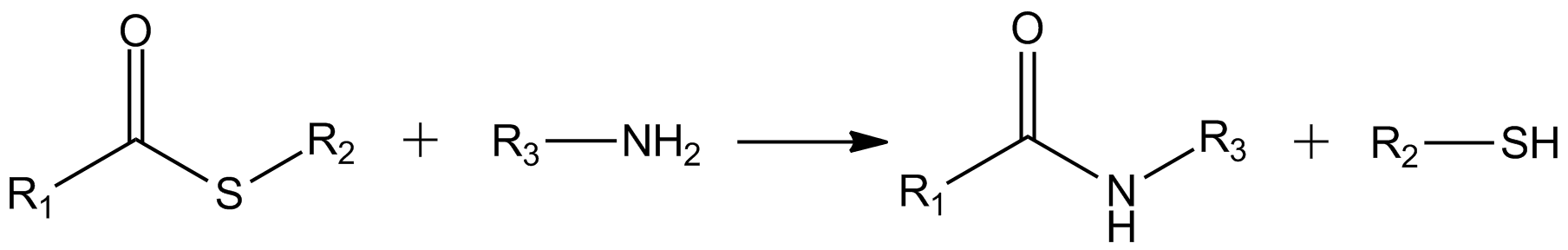
Thioester
In organic chemistry, thioesters are organosulfur compounds with the molecular structure . They are analogous to carboxylate esters () with the sulfur in the thioester replacing oxygen in the carboxylate ester, as implied by the thio- prefix. They are the product of esterification of a carboxylic acid () with a thiol (). In biochemistry, the best-known thioesters are derivatives of coenzyme A, e.g., acetyl-CoA.Matthys J. Janssen "Carboxylic Acids and Esters" in PATAI's Chemistry of Functional Groups: Carboxylic Acids and Esters, Saul Patai, Ed. John Wiley, 1969, New York: pp. 705–764. The R and R' represent organyl groups, or H in the case of R. Synthesis One route to thioesters involves the reaction of an acid chloride with an alkali metal salt of a thiol: : Another common route entails the displacement of halides by the alkali metal salt of a thiocarboxylic acid. For example, thioacetate esters are commonly prepared by alkylation of potassium thioacetate: : Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Decarboxylating Reduction
Decarboxylation is a chemical reaction that removes a carboxyl group and releases carbon dioxide (CO2). Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is the first chemical step in photosynthesis, is called carboxylation, the addition of CO2 to a compound. Enzymes that catalyze decarboxylations are called decarboxylases or, the more formal term, carboxy-lyases ( EC number 4.1.1). In organic chemistry The term "decarboxylation" usually means replacement of a carboxyl group () with a hydrogen atom: : Decarboxylation is one of the oldest known organic reactions. It is one of the processes assumed to accompany pyrolysis and destructive distillation. Overall, decarboxylation depends upon stability of the carbanion synthon , although the anion may not be a true chemical intermediate. Typically, carboxylic acids decarboxylate slowly, but carboxylic acids with an α electron-withdrawing group ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
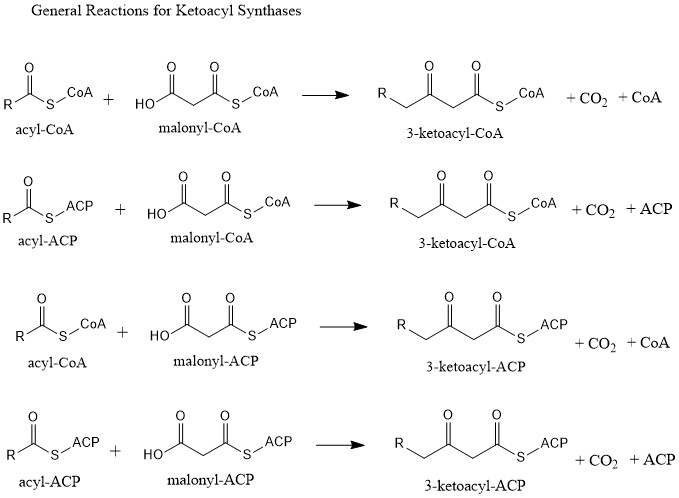
Ketoacyl Synthase
Ketoacyl synthases (KSs) catalyze the condensation reaction of acyl-CoA or acyl-acyl ACP with malonyl-CoA to form 3-ketoacyl-CoA or with malonyl-ACP to form 3-ketoacyl-ACP. This reaction is a key step in the fatty acid synthesis cycle, as the resulting acyl chain is two carbon atoms longer than before. KSs exist as individual enzymes, as they do in type II fatty acid synthesis and type II polyketide synthesis, or as domains in large multidomain enzymes, such as type I fatty acid synthases (FASs) and polyketide synthases (PKSs). KSs are divided into five families: KS1, KS2, KS3, KS4, and KS5. Multidomain enzyme systems Fatty acid synthase Fatty acid synthase (FAS) is the enzyme system involved in de novo fatty acid synthesis. FAS is an iterative multienzyme consisting of several component enzymes, one of which is ketoacyl synthase. There are two types of FASs: type I and type II. Type I FASs are highly integrated multidomain enzymes. They contain discrete functional domains resp ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Pyruvic Acid
Pyruvic acid (CH3COCOOH) is the simplest of the keto acids, alpha-keto acids, with a carboxylic acid and a ketone functional group. Pyruvate, the conjugate acid, conjugate base, CH3COCOO−, is an metabolic intermediate, intermediate in several metabolic pathways throughout the cell. Pyruvic acid can be made from glucose through glycolysis, converted back to carbohydrates (such as glucose) via gluconeogenesis, or converted to fatty acids through a reaction with acetyl-CoA. It can also be used to construct the amino acid alanine and can be converted into ethanol or lactic acid via fermentation. Pyruvic acid supplies energy to cell (biology), cells through the citric acid cycle (also known as the Krebs cycle) when oxygen is present (aerobic respiration), and alternatively lactic acid fermentation, ferments to produce lactic acid, lactate when oxygen is lacking. Chemistry In 1834, Théophile-Jules Pelouze distilled tartaric acid and isolated glutaric acid and another unknown organi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Oxygenase
An oxygenase is any enzyme that oxidizes a substrate by transferring the oxygen from molecular oxygen O2 (as in air) to it. The oxygenases form a class of oxidoreductases; their EC number is EC 1.13 or EC 1.14. Structure Most oxygenases contain either a metal, usually iron, or an organic cofactor, usually flavin. These cofactors interact with O2, leading to its transfer to substrate. Oxygenases constitute a major intracellular source of iron and carbon monoxide Mechanism Two types of oxygenases are recognized: *Monooxygenases, or mixed function oxidase, transfer one oxygen atom to the substrate, and reduce the other oxygen atom to water. *Dioxygenases, or oxygen transferases, incorporate both atoms of molecular oxygen (O2) into the product(s) of the reaction. Among the most common monooxygenases are the cytochrome P450 oxidase Cytochromes P450 (P450s or CYPs) are a superfamily of enzymes containing heme as a cofactor that mostly, but not exclusively, function as m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |