|
Alpha-keratin
Alpha-keratin, or α-keratin, is a type of keratin found in mammalian vertebrates. This protein is the primary component in hairs, horn (anatomy), horns, claws, Nail (anatomy), nails and the Epidermis, epidermis layer of the skin. α-keratin is a Scleroprotein, fibrous structural protein, meaning it is made up of amino acids that form a repeating Protein secondary structure, secondary structure. The secondary structure of α-keratin is very similar to that of a traditional protein Alpha helix, α-helix and forms a coiled coil. Due to its tightly wound structure, it can function as one of the strongest biological materials and has various functions in mammals, from Predation, predatory claws to hair for warmth. α-keratin is synthesized through protein biosynthesis, utilizing Transcription (biology), transcription and Translation (biology), translation, but as the cell matures and is full of α-keratin, it dies, creating a strong non-Blood vessel, vascular unit of keratinized tissue. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Keratin
Keratin () is one of a family of structural fibrous proteins also known as ''scleroproteins''. It is the key structural material making up Scale (anatomy), scales, hair, Nail (anatomy), nails, feathers, horn (anatomy), horns, claws, Hoof, hooves, and the outer layer of skin in vertebrates. Keratin also protects epithelial cells from damage or stress. Keratin is extremely insoluble in water and organic solvents. Keratin monomers assemble into bundles to form intermediate filaments, which are tough and form strong mineralization (biology), unmineralized epidermal appendages found in reptiles, birds, amphibians, and mammals. Excessive keratinization participate in fortification of certain tissues such as in horns of cattle and rhinos, and armadillos' osteoderm. The only other biology, biological matter known to approximate the toughness of keratinized tissue is chitin. Keratin comes in two types: the primitive, softer forms found in all vertebrates and the harder, derived forms fou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
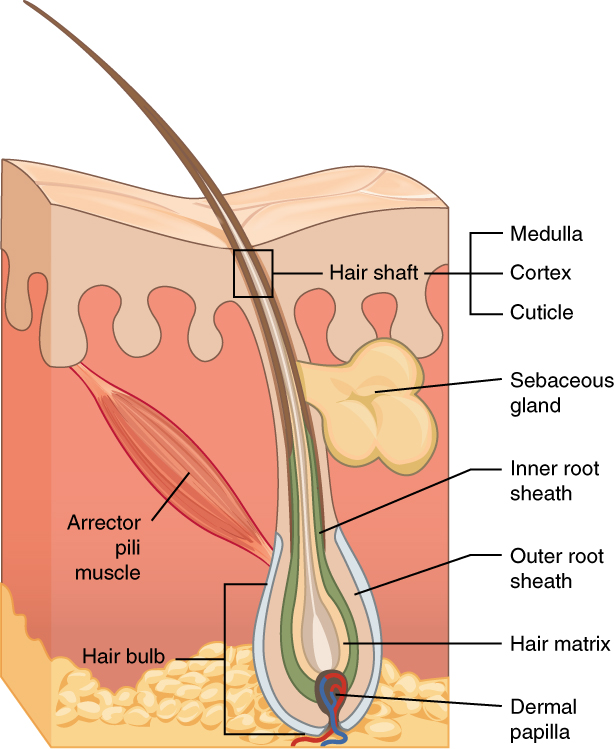
Hair
Hair is a protein filament that grows from follicles found in the dermis. Hair is one of the defining characteristics of mammals. The human body, apart from areas of glabrous skin, is covered in follicles which produce thick terminal and fine vellus hair. Most common interest in hair is focused on hair growth, hair types, and hair care, but hair is also an important biomaterial primarily composed of protein, notably alpha-keratin. Attitudes towards different forms of hair, such as hairstyles and hair removal, vary widely across different cultures and historical periods, but it is often used to indicate a person's personal beliefs or social position, such as their age, gender, or religion. Overview Meaning The word "hair" usually refers to two distinct structures: #the part beneath the skin, called the hair follicle, or, when pulled from the skin, the bulb or root. This organ is located in the dermis and maintains stem cells, which not only re-grow the hair afte ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Keratin Creation Red
Keratin () is one of a family of structural fibrous proteins also known as ''scleroproteins''. It is the key structural material making up scales, hair, nails, feathers, horns, claws, hooves, and the outer layer of skin in vertebrates. Keratin also protects epithelial cells from damage or stress. Keratin is extremely insoluble in water and organic solvents. Keratin monomers assemble into bundles to form intermediate filaments, which are tough and form strong unmineralized epidermal appendages found in reptiles, birds, amphibians, and mammals. Excessive keratinization participate in fortification of certain tissues such as in horns of cattle and rhinos, and armadillos' osteoderm. The only other biological matter known to approximate the toughness of keratinized tissue is chitin. Keratin comes in two types: the primitive, softer forms found in all vertebrates and the harder, derived forms found only among sauropsids (reptiles and birds). Spider silk is classified as keratin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Translation (biology)
In biology, translation is the process in living Cell (biology), cells in which proteins are produced using RNA molecules as templates. The generated protein is a sequence of amino acids. This sequence is determined by the sequence of nucleotides in the RNA. The nucleotides are considered three at a time. Each such triple results in the addition of one specific amino acid to the protein being generated. The matching from nucleotide triple to amino acid is called the genetic code. The translation is performed by a large complex of functional RNA and proteins called ribosomes. The entire process is called gene expression. In translation, messenger RNA (mRNA) is decoded in a ribosome, outside the nucleus, to produce a specific amino acid chain, or polypeptide. The polypeptide later protein folding, folds into an Activation energy, active protein and performs its functions in the cell. The polypeptide can also start folding during protein synthesis. The ribosome facilitates decoding ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Polymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many forms of polymerization and different systems exist to categorize them. In chemical compounds, polymerization can occur via a variety of reaction mechanisms that vary in complexity due to the functional groups present in the reactants and their inherent steric effects. In more straightforward polymerizations, alkenes form polymers through relatively simple radical reactions; in contrast, reactions involving substitution at a carbonyl group require more complex synthesis due to the way in which reactants polymerize. As alkenes can polymerize in somewhat straightforward radical reactions, they form useful compounds such as polyethylene and polyvinyl chloride (PVC), which are produced in high tonnages each year due to their usefulnes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-terminus
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the amine group is bonded to the carboxylic group of another amino acid, making it a chain. That leaves a free carboxylic group at one end of the peptide, called the C-terminus, and a free amine group on the other end called the N-terminus. By convention, peptide sequences are written N-terminus to C-terminus, left to right (in LTR writing systems). This correlates the translation direction to the text direction, because when a protein is translated from messenger RNA, it is created from the N-terminus to the C-terminus, as amino acids are added to the carboxyl end of the protein. Chemistry Each amino acid has an amine group and a carboxylic group. Amino acids link to one another by peptide bonds which form through a dehydration reaction that ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
C-terminus
The C-terminus (also known as the carboxyl-terminus, carboxy-terminus, C-terminal tail, carboxy tail, C-terminal end, or COOH-terminus) is the end of an amino acid chain (protein Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ... or polypeptide), terminated by a free carboxyl group (-COOH). When the protein is translated from messenger RNA, it is created from N-terminus to C-terminus. The convention for writing peptide sequences is to put the C-terminal end on the right and write the sequence from N- to C-terminus. Chemistry Each amino acid has a carboxyl group and an amine group. Amino acids link to one another to form a chain by a dehydration reaction which joins the amine group of one amino acid to the carboxyl group of the next. Thus polypeptide chains have an end with an ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Disulfide
In chemistry, a disulfide (or disulphide in British English) is a compound containing a functional group or the anion. The linkage is also called an SS-bond or sometimes a disulfide bridge and usually derived from two thiol groups. In inorganic chemistry, the anion appears in a few rare minerals, but the functional group has tremendous importance in biochemistry. Disulfide bridges formed between thiol groups in two cysteine residues are an important component of the tertiary and quaternary structure of proteins. Compounds of the form are usually called ''persulfides'' instead. Organic disulfides Structure Disulfides have a C–S–S–C dihedral angle approaching 90°. The S–S bond length is 2.03 Å in diphenyl disulfide, similar to that in elemental sulfur. Disulfides are usually symmetric but they can also be unsymmetric. Symmetrical disulfides are compounds of the formula . Most disulfides encountered in organosulfur chemistry are symmetrical disulfides. Unsymme ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimer (chemistry)
In chemistry, dimerization is the process of joining two identical or similar Molecular entity, molecular entities by Chemical bond, bonds. The resulting bonds can be either strong or weak. Many symmetrical chemical species are described as dimers, even when the monomer is unknown or highly unstable. The term ''homodimer'' is used when the two subunits are identical (e.g. A–A) and ''heterodimer'' when they are not (e.g. A–B). The reverse of dimerization is often called Dissociation (chemistry), dissociation. When two oppositely-charged ions associate into dimers, they are referred to as ''Bjerrum pairs'', after Danish chemist Niels Bjerrum. Noncovalent dimers Anhydrous carboxylic acids form dimers by hydrogen bonding of the acidic hydrogen and the carbonyl oxygen. For example, acetic acid forms a dimer in the gas phase, where the monomer units are held together by hydrogen bonds. Many OH-containing molecules form dimers, e.g. the water dimer. Dimers that form based on w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cysteine
Cysteine (; symbol Cys or C) is a semiessential proteinogenic amino acid with the chemical formula, formula . The thiol side chain in cysteine enables the formation of Disulfide, disulfide bonds, and often participates in enzymatic reactions as a nucleophile. Cysteine is chiral, but both D and L-cysteine are found in nature. LCysteine is a protein monomer in all biota, and D-cysteine acts as a signaling molecule in mammalian nervous systems. Cysteine is named after its discovery in urine, which comes from the urinary bladder or cyst, from Ancient Greek, Greek κύστις ''kýstis'', "bladder". The thiol is susceptible to oxidation to give the disulfide bond, disulfide derivative cystine, which serves an important structural role in many proteins. In this case, the symbol Cyx is sometimes used. The deprotonated form can generally be described by the symbol Cym as well. When used as a food additive, cysteine has the E number E920. Cysteine is Genetic code, encoded by the codo ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arginine
Arginine is the amino acid with the formula (H2N)(HN)CN(H)(CH2)3CH(NH2)CO2H. The molecule features a guanidinium, guanidino group appended to a standard amino acid framework. At physiological pH, the carboxylic acid is deprotonated (−CO2−) and both the amino and guanidino groups are protonated, resulting in a cation. Only the -arginine (symbol Arg or R) enantiomer is found naturally. Arg residues are common components of proteins. It is Genetic code, encoded by the DNA codon table, codons CGU, CGC, CGA, CGG, AGA, and AGG. The guanidine group in arginine is the Precursor (chemistry), precursor for the biosynthesis of nitric oxide. Like all amino acids, it is a white, water-soluble solid. The one-letter symbol R was assigned to arginine for its phonetic similarity. History Arginine was first isolated in 1886 from Lupinus luteus, yellow lupin seedlings by the German chemist Ernst Schulze (chemist), Ernst Schulze and his assistant Ernst Steiger. He named it from the Greek ''árg ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Leucine
Leucine (symbol Leu or L) is an essential amino acid that is used in the biosynthesis of proteins. Leucine is an α-amino acid, meaning it contains an α-amino group (which is in the protonated −NH3+ form under biological conditions), an α-Carboxylic acid, carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and a side chain Isobutyl, isobutyl group, making it a Chemical polarity, non-polar Aliphatic compound, aliphatic amino acid. It is Essential amino acid, essential in humans, meaning the body cannot synthesize it; it must be obtained from the diet. Human dietary sources are foods that contain protein, such as meats, dairy products, soy products, and beans and other legumes. It is genetic code, encoded by the codons UUA, UUG, CUU, CUC, CUA, and CUG. Leucine is named after the Greek language, Greek word for "white": ''λευκός'' (''leukós'', "white"), after its common appearance as a white powder, a property it shares with many ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |