|
Alkynylation
In organic chemistry, alkynylation is an addition reaction in which a terminal alkyne () is added to a carbonyl group () to form an α-alkynyl alcohol (). When the acetylide is formed from acetylene (), the reaction gives an α-ethynyl alcohol. This process is often referred to as ethynylation. Such processes often involve metal acetylide intermediates. Scope The principal reaction of interest involves the addition of the acetylene () to a ketone () or aldehyde (): :RR'C=O + HC#CR'' -> RR'C(OH)C#CR'' The reaction proceeds with retention of the triple bond. For aldehydes and unsymmetrical ketones, the product is chiral, hence there is interest in asymmetric variants. These reactions invariably involve metal-acetylide intermediates. This reaction was discovered by chemist John Ulric Nef in 1899 while experimenting with reactions of elemental sodium, phenylacetylene, and acetophenone. For this reason, the reaction is sometimes referred to as Nef synthesis. Sometimes this rea ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Propargyl Alcohol
Propargyl alcohol, or 2-propyn-1-ol, is an organic compound with the formula C3H4O. It is the simplest stable alcohol containing an alkyne functional group. Propargyl alcohol is a colorless viscous liquid that is miscible with water and most polar organic solvents. Reactions and applications Propargyl alcohol polymerizes with heating or treatment with base. It is used as a corrosion inhibitor, a metal complex solution, a solvent stabilizer and an electroplating brightener additive. It is also used as an intermediate in organic synthesis. Secondary and tertiary substituted propargylic alcohols undergo catalyzed rearrangement reactions to form α,β-unsaturated carbonyl compounds via the Meyer–Schuster rearrangement and others. It can be oxidized to propynal or propargylic acid. As an indication of the electronegativity of an sp carbon, propargyl alcohol is significantly more acidic (p''K''a = 13.6) compared to its sp2-containing analog allyl alcohol (p''K''a = 15.5), w ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Organic Chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain carbon atoms.Clayden, J.; Greeves, N. and Warren, S. (2012) ''Organic Chemistry''. Oxford University Press. pp. 1–15. . Study of structure determines their structural formula. Study of properties includes Physical property, physical and Chemical property, chemical properties, and evaluation of Reactivity (chemistry), chemical reactivity to understand their behavior. The study of organic reactions includes the organic synthesis, chemical synthesis of natural products, drugs, and polymers, and study of individual organic molecules in the laboratory and via theoretical (in silico) study. The range of chemicals studied chemistry includes hydrocarbons (compounds containing only carbon and hydrogen) as well as compounds based on carbon, but a ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Nef Synthesis
In organic chemistry, Nef synthesis is the addition of sodium acetylides to aldehydes and ketones to yield propargyl alcohols. It is named for John Ulric Nef (chemist), John Ulric Nef, who discovered the reaction in 1899. : This process is often erroneously referred to as the Nef reaction, which is an unrelated chemical transformation discovered by the same chemist. See also *Favorskii reaction *Alkynation References Carbon-carbon bond forming reactions Organometallic chemistry Addition reactions Name reactions {{organic-chem-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Lithium Amide
Lithium amide or lithium azanide is an inorganic compound with the chemical formula . It is a white solid with a tetragonal crystal structure. Lithium amide can be made by treating lithium metal with liquid ammonia: : Lithium amide decomposes into ammonia and lithium imide upon heating. Applications Lithium amide, when mixed with lithium hydride, shows applications in hydrogen storage.The reaction begins with lithium amide's decomposition into ammonia and lithium imide. Lithium hydride then deprotonates ammonia to form lithium amide. The reverse reaction can occur between hydrogen and the lithium imide side product. Other lithium amides The conjugate bases of amines are known as amides. Thus, a ''lithium amide'' may also refer to any compound in the class of the lithium salt of an amine. These compounds have the general form , with the chemical lithium amide itself as the parent structure. Common lithium amides include lithium diisopropylamide (LDA), lithium tetramethylpi ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Grignard Reagent
Grignard reagents or Grignard compounds are chemical compounds with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromide . They are a subclass of the organomagnesium compounds. Grignard compounds are popular reagents in organic synthesis for creating new carbon–carbon bonds. For example, when reacted with another halogenated compound in the presence of a suitable catalyst, they typically yield and the magnesium halide as a byproduct; and the latter is insoluble in the solvents normally used. Grignard reagents are rarely isolated as solids. Instead, they are normally handled as solutions in solvents such as diethyl ether or tetrahydrofuran using air-free techniques. Grignard reagents are complex with the magnesium atom bonded to two ether ligands as well as the halide and organyl ligands. The discovery of the Grignard reaction in 1900 was recogn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Acetylide Carbonyl Addition
In chemistry, an acetylide is a compound that can be viewed as the result of replacing one or both hydrogen atoms of acetylene (ethyne) by metallic or other cations. Calcium carbide is an important industrial compound, which has long been used to produce acetylene for welding and illumination. It is also a major precursor to vinyl chloride. Other acetylides are reagents in organic synthesis. Nomenclature The term acetylide is used loosely. It apply to an acetylene , where R = H or a side chain that is usually organic. The nomenclature can be ambiguous with regards to the distinction between compounds of the type and . When both hydrogens of acetylene are replaced by metals, the compound can also be called carbide, e.g. calcium carbide , which is calcium acetylide. When only one hydrogen atom is replaced, the anion may be called hydrogen acetylide or the prefix ''mono''- may be attached to the metal, as in monosodium acetylide or sodium hydrogen acetylide, . An acetylide may be ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Acetic Acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main component of vinegar apart from water. Historically, vinegar was produced from the third century BC and was likely the first acid to be produced in large quantities. Acetic acid is the second simplest carboxylic acid (after formic acid). It is an important Reagent, chemical reagent and industrial chemical across various fields, used primarily in the production of cellulose acetate for photographic film, polyvinyl acetate for wood Adhesive, glue, and synthetic fibres and fabrics. In households, diluted acetic acid is often used in descaling agents. In the food industry, acetic acid is controlled by the E number, food additive code E260 as an acidity regulator and as a condiment. In biochemistry, the acetyl group, derived from acetic acid, is funda ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
Alkoxy Group
In chemistry, the alkoxy group is an alkyl group which is Single bond, singularly bonded to oxygen; thus . Denoted usually with apostrophe('). The range of alkoxy groups is vast, the simplest being methoxy (). An ethoxy group () is found in the organic compound ethyl phenyl ether (, also known as ethoxybenzene). Related to alkoxy groups are aryloxy groups, which have an aryl group singularly bonded to oxygen such as the phenoxy group (). An alkoxy or aryloxy group bonded to an alkyl or aryl () is an ether. If bonded to H it is an Alcohol (chemistry), alcohol. The term ''alkoxide'' refers to the anionic conjugate bases of alcohols () or to ionic compounds containing such an anion. Alkoxide compounds are derivatives of alcohols where the hydrogen of the –OH group is replaced by a metal; for example, the sodium salt (chemistry), salt of ethanol () is sodium ethoxide, containing ethoxide anions and sodium cations . References {{DEFAULTSORT:Alkoxy Group Alkoxy groups ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |
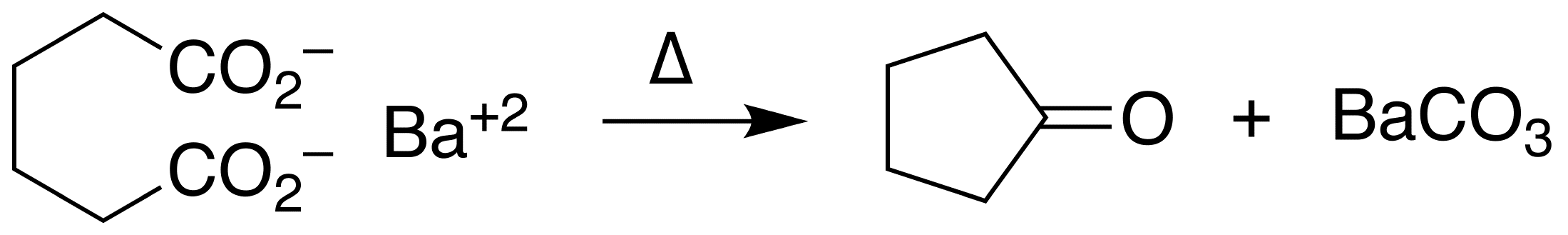
Cyclopentanone
Cyclopentanone is the organic compound with the formula (CH2)4CO. This cyclic ketone is a colorless volatile liquid. Preparation Ketonic decarboxylation of adipic acid gives cyclopentanone. The reaction is conducted at elevated temperatures in the presence of barium hydroxide. : The Pd-catalyzed oxidation of cyclopentene also gives cyclopentanone. Uses Cyclopentanone is common precursor to fragrances, especially those related to jasmine and jasmone. Examples include 2-pentyl- and 2-heptylcyclopentanone. It is a versatile synthetic intermediate, being a precursor to cyclopentobarbital. Cyclopentanone is also used to make cyclopentamine, the pesticide pencycuron, and pentethylcyclanone. It is also used as a precursor to cubane-1,4-dicarboxylate, which is used to synthesize other substituted cubanes, such as the high explosives heptanitrocubane and octanitrocubane. References {{Authority control Cycloalkanones, 5 Ketone solvents Perfume ingredients Cyclopentanes ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] [Amazon] |