|
4-Dimethylamino-4-(p-tolyl)cyclohexanone
4-Dimethylamino-4-(''p''-tolyl)cyclohexanone (sometimes known as dimetamine) is a opioid analgesic with an arylcyclohexylamine chemical structure. It was developed by Daniel Lednicer at Upjohn in the 1970s. It has around the same analgesic potency as morphine, with analogues where the ''para''-methyl group is replaced by a halogen being slightly weaker. Derivatives where the ketone group has been reacted with a Grignard reagent to add a phenethyl side chain are several hundred times stronger (as is seen in the compound BDPC). Legal Status 4-Dimethylamino-4-(p-tolyl)cyclohexanone is specifically listed as an illegal drug in Latvia. It is also covered by drug analogue laws in various jurisdictions as a generic arylcyclohexylamine derivative. See also * 3-HO-PCP * 4-Keto-PCP * Tramadol Tramadol, sold under the brand name Tramal among others, is an opioid analgesic, pain medication and a serotonin–norepinephrine reuptake inhibitor (SNRI) used to treat moderately severe pa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
BDPC
BDPC (systematic name 4-(4-bromophenyl)-4-(dimethylamino)-1-(2-phenylethyl)cyclohexanol; also known as bromadol) is a potent fully synthetic opioid with a distinctive arylcyclohexylamine chemical structure. It was developed by Daniel Lednicer at Upjohn in the 1970s. Initial studies estimated that it was around 10,000 times the potency of morphine in animal models. However, later studies using more modern techniques assigned a value of 504 times the potency of morphine for the more active ''trans''-isomer. This drug was first seized along with three kilograms of acetylfentanyl in an April 25, 2013 police action in Montreal, Canada, and has reportedly continued to be available on the designer drug market internationally. Analogues where the ''para''-bromine is replaced by chlorine or a methyl group retain similar activity, while the ''meta''-hydroxyl derivative demonstrated robust antagonist activity. ] ] See also * 3-HO-PCP * 4-Keto-PCP * C-8813 * Cebranopadol * Ciramadol * ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
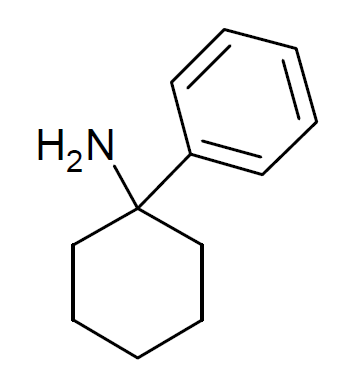
Arylcyclohexylamine
Arylcyclohexylamines, also known as arylcyclohexamines or arylcyclohexanamines, are a chemical class of pharmaceutical, designer, and experimental drugs. History Phencyclidine (PCP) is believed to be the first arylcyclohexylamine with recognized anesthetic properties, but several arylcyclohexylamines were described before PCP in the scientific literature, beginning with PCA (1-phenylcyclohexan-1-amine) the synthesis of which was first published in 1907. PCP itself was discovered in 1926 but not researched by the pharmaceutical industry until the 1950s. PCE was reported in 1953 and PCMo (4-(1-phenyl-cyclohexyl)-morpholine see chart below for figure) in 1954, with PCMo described as a potent sedative. Arylcyclohexylamine anesthetics were intensively investigated at Parke-Davis, beginning with the 1956 studies of PCP and later the related compound ketamine. The 1970s saw the debut of these compounds, especially PCP and its analogues, as illicitly used recreational drugs due to th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Arylcyclohexylamines
Arylcyclohexylamines, also known as arylcyclohexamines or arylcyclohexanamines, are a chemical class of pharmaceutical drug, pharmaceutical, designer drug, designer, and experimental drugs. History Phencyclidine (PCP) is believed to be the first arylcyclohexylamine with recognized anesthetic properties, but several arylcyclohexylamines were described before PCP in the scientific literature, beginning with PCA (1-phenylcyclohexan-1-amine) the synthesis of which was first published in 1907. PCP itself was discovered in 1926 but not researched by the pharmaceutical industry until the 1950s. Eticyclidine, PCE was reported in 1953 and PCMo (4-(1-phenyl-cyclohexyl)-morpholine see chart below for figure) in 1954, with PCMo described as a potent sedative. Arylcyclohexylamine anesthetics were intensively investigated at Parke-Davis, beginning with the 1956 studies of PCP and later the related compound ketamine. The 1970s saw the debut of these compounds, especially PCP and its structura ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
4-Keto-PCP
4-Keto-PCP is a recreational designer drug from the arylcyclohexylamine family, with dissociative effects. It has potency in between that of ketamine and phencyclidine but with somewhat more sedating effects in animal studies. See also * 3-HO-PCP * 3-Fluoro-PCP * Bromadol * Dimetamine * Methoxetamine Methoxetamine (MXE) is a dissociative hallucinogen that has been sold as a designer drug. It differs from many dissociatives such as ketamine and phencyclidine (PCP) that were developed as pharmaceutical drugs for use as general anesthetics in ... References Arylcyclohexylamines Designer drugs Dissociative drugs 1-Piperidinyl compounds {{nervous-system-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
3-HO-PCP
3-Hydroxyphencyclidine (3-HO-PCP) is a dissociative of the arylcyclohexylamine class related to phencyclidine (PCP) that has been sold online as a designer drug. Pharmacology 3-HO-PCP acts as a high-affinity uncompetitive antagonist of the NMDA receptor via the dizocilpine (MK-801) site (Ki = 30 nM). It has much higher affinity than PCP for this site (Ki = 250 nM, for comparison; 8-fold difference). The drug also has high affinity for the μ-opioid receptor (MOR) (Ki = 39–60 nM) in animal test subjects, the κ-opioid receptor (KOR) (Ki = 140 nM), and the sigma σ1 receptor (Ki = 42 nM; IC50 = 19 nM), whereas it has only low affinity for the δ-opioid receptor (Ki = 2,300 nM). The high affinity of 3-HO-PCP for opioid receptors is unique among arylcyclohexylamines and is in contrast to PCP, which has only very low affinity for the MOR (Ki = 11,000–26,000 nM; 282- to 433-fold difference) and the other opioid receptors (Ki = 4,100 ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Propylhexedrine
Propylhexedrine, sold under the brand name Benzedrex among others, is an alkylamine primarily utilized as a topical nasal decongestant. Its main indications are relief of congestion due to colds, allergies, and allergic rhinitis. Propylhexedrine was first used medically in 1949, with the release of Benzedrex by Smith, Kline & French, and it has been used, mainly within the United States, since then. Medical use Propylhexedrine is used to treat acute nasal congestion related to the common cold, allergies, and hay fever. For nasal congestion, the dosage is listed as four inhalations (two inhalations per nostril) every two hours for adults and children 6–12 years of age. Each inhalation delivers 0.4 to 0.5 mg (400 to 500 ''μ''g) in 800 mL of air. Use is not to exceed three days. Historically, it has also been used for weight loss in oral tablet preparations at a dose of 25 mg. No medications containing propylhexedrine are currently approved for weight loss in any country sin ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Dimethylamino Compounds
Dimethylamine is an organic compound with the formula (CH3)2NH. This secondary amine is a colorless, flammable gas with an ammonia-like odor. Dimethylamine is commonly encountered commercially as a solution in water at concentrations up to around 40%. An estimated 270,000 tons were produced in 2005. Structure and synthesis The molecule consists of a nitrogen atom with two methyl substituents and one hydrogen. Dimethylamine is a base (chemistry), weak base and the pKa of the ammonium CH3--CH3 is 10.73, a value above methylamine (10.64) and trimethylamine (9.79). Dimethylamine reacts with acids to form salts, such as dimethylamine hydrochloride, an odorless white solid with a melting point of 171.5 °C. Dimethylamine is produced by catalytic reaction of methanol and ammonia Ammonia is an inorganic chemical compound of nitrogen and hydrogen with the chemical formula, formula . A Binary compounds of hydrogen, stable binary hydride and the simplest pnictogen hydride, ammoni ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tramadol
Tramadol, sold under the brand name Tramal among others, is an opioid analgesic, pain medication and a serotonin–norepinephrine reuptake inhibitor (SNRI) used to treat moderately severe pain. When taken by mouth in an immediate-release formulation, the onset of pain relief usually begins within an hour. It is also available by injection. It is available in combination with paracetamol (acetaminophen). As is typical of opioids, common side effects include constipation, pruritis, itchiness, and nausea. Serious side effects may include hallucinations, seizures, increased risk of serotonin syndrome, decreased alertness, and drug addiction. A change in dosage may be recommended in those with chronic kidney disease, kidney or liver problems. It is not recommended in those who are at risk of suicide or in those who are pregnant. While not recommended in women who are breastfeeding, those who take a single dose should not generally have to stop breastfeeding. Tramadol is converted ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Journal Of Medicinal Chemistry
The ''Journal of Medicinal Chemistry'' is a biweekly peer-reviewed medical journal covering research in medicinal chemistry. It is published by the American Chemical Society. It was established in 1959 as the ''Journal of Medicinal and Pharmaceutical Chemistry'' and obtained its current name in 1963. Philip S. Portoghese served as editor-in-chief from 1972 to 2011. In 2012, Gunda Georg (University of Minnesota) and Shaomeng Wang (University of Michigan) succeeded Portoghese (University of Minnesota). In 2021, Craig W. Lindsley (Vanderbilt University) became editor-in-chief. According to the ''Journal Citation Reports'', the journal has a 2023 impact factor of 7.1. See also *ACS Medicinal Chemistry Letters ''ACS Medicinal Chemistry Letters'' is a monthly peer-reviewed scientific journal covering medicinal chemistry. It was established in 2009 and is published by the American Chemical Society. The editor-in-chief is Dennis C. Liotta (Emory University ... References External ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Grignard Reagent
Grignard reagents or Grignard compounds are chemical compounds with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromide . They are a subclass of the organomagnesium compounds. Grignard compounds are popular reagents in organic synthesis for creating new carbon–carbon bonds. For example, when reacted with another halogenated compound in the presence of a suitable catalyst, they typically yield and the magnesium halide as a byproduct; and the latter is insoluble in the solvents normally used. Grignard reagents are rarely isolated as solids. Instead, they are normally handled as solutions in solvents such as diethyl ether or tetrahydrofuran using air-free techniques. Grignard reagents are complex with the magnesium atom bonded to two ether ligands as well as the halide and organyl ligands. The discovery of the Grignard reaction in 1900 was recogn ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Opioid Analgesic
Opioids are a class of drugs that derive from, or mimic, natural substances found in the opium poppy plant. Opioids work on opioid receptors in the brain and other organs to produce a variety of morphine-like effects, including pain relief. The terms "opioid" and "opiate" are sometimes used interchangeably, but the term "opioid" is used to designate all substances, both natural and synthetic, that bind to opioid receptors in the brain. Opiates are alkaloid compounds naturally found in the opium poppy plant ''Papaver somniferum''. Medically they are primarily used for pain relief, including anesthesia. Other medical uses include suppression of diarrhea, replacement therapy for opioid use disorder, and suppressing cough. The opioid receptor antagonist naloxone is used to reverse opioid overdose. Extremely potent opioids such as carfentanil are approved only for veterinary use. Opioids are also frequently used recreationally for their euphoric effects or to prevent withdrawal. ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Halogen
The halogens () are a group in the periodic table consisting of six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and the radioactive elements astatine (At) and tennessine (Ts), though some authors would exclude tennessine as its chemistry is unknown and is theoretically expected to be more like that of gallium. In the modern IUPAC nomenclature, this group is known as group 17. The word "halogen" means "salt former" or "salt maker". When halogens react with metals, they produce a wide range of salts, including calcium fluoride, sodium chloride (common table salt), silver bromide and potassium iodide. The group of halogens is the only periodic table group that contains elements in three of the main states of matter at standard temperature and pressure, though not far above room temperature the same becomes true of groups 1 and 15, assuming white phosphorus is taken as the standard state.This could also be the case for group 12, al ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |