|
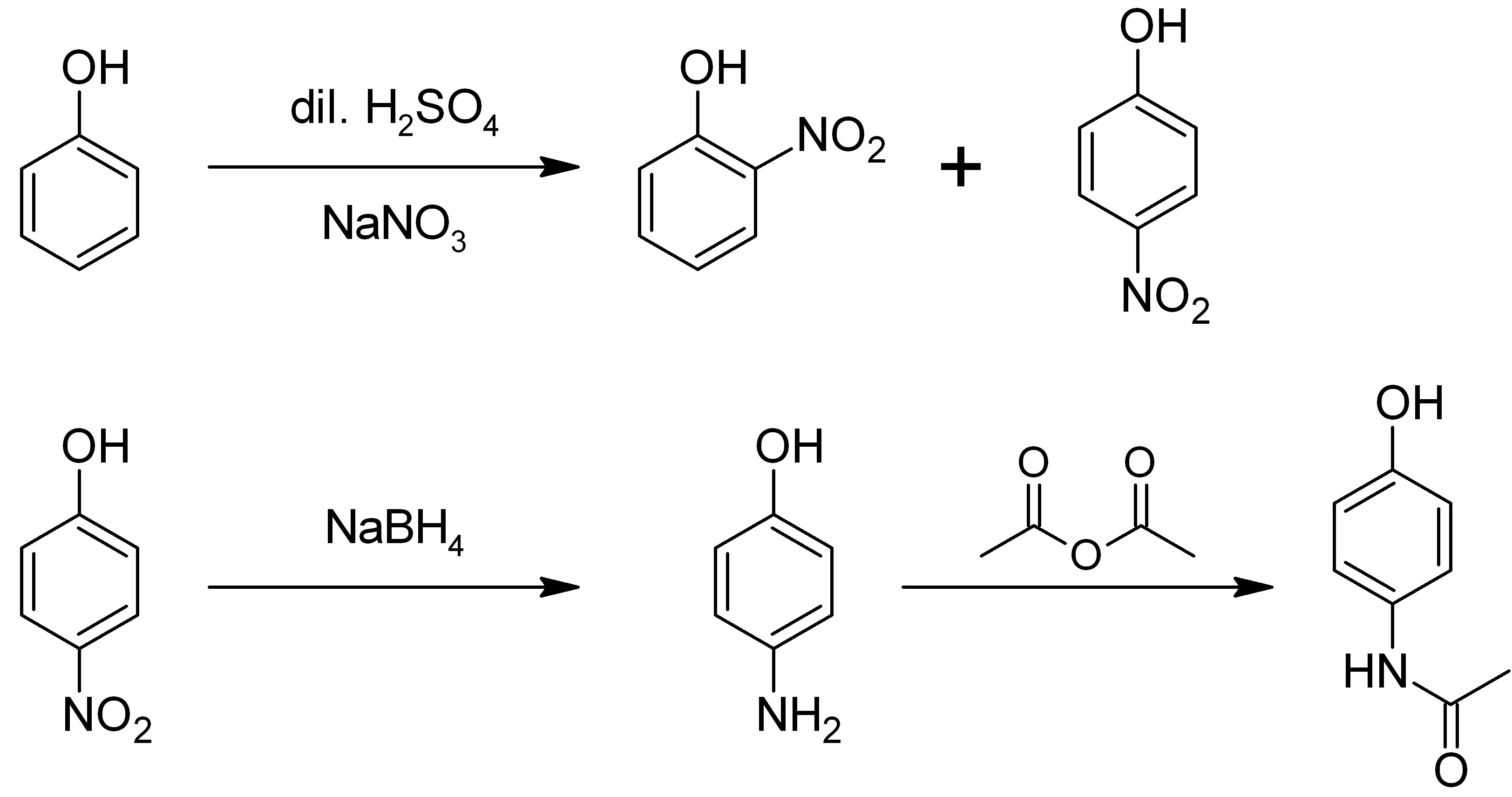
4-aminophenol
4-Aminophenol (or ''para''-aminophenol or ''p''-aminophenol) is an organic compound with the formula H2NC6H4OH. Typically available as a white powder, it is commonly used as a developer for black-and-white film, marketed under the name Rodinal. Reflecting its slightly hydrophilic character, the white powder is moderately soluble in alcohols and can be recrystallized from hot water. In the presence of a base, it oxidizes readily. The methylated derivatives ''N''-methylaminophenol and ''N'',''N''-dimethylaminophenol are of commercial value. The compound is one of three isomeric aminophenols, the other two being 2-aminophenol and 3-aminophenol. __TOC__ Preparation From phenol It is produced from phenol by nitration followed by reduction with iron. Alternatively, the partial hydrogenation of nitrobenzene affords phenylhydroxylamine, which rearranges primarily to 4-aminophenol ( Bamberger rearrangement). :C6H5NO2 + 2 H2 → C6H5NHOH + H2O :C6H5NHOH → HOC6H4NH2 From ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2-aminophenol
2-Aminophenol is an organic compound with the formula C6H7NO. Along with its isomer 4-aminophenol, it is an amphoteric molecule and a reducing agent. It is a useful reagent for the synthesis of dyes and heterocyclic compounds.Mitchell, S.C. & Waring, R.H. "Aminophenols." In Ullmann’s Encyclopedia of Industrial Chemistry; 2002 Wiley-VCH, . Reflecting its slight hydrophilic character, white powder is moderately soluble in alcohols and can be recrystallized from hot water. Synthesis and structure 2-Aminophenol (and its isomer, 4-aminophenol) is industrially synthesized by reducing the corresponding nitrophenol by hydrogen in the presence of various catalysts. The nitrophenols can also be reduced with iron. The compound exhibits intra- and intermolecular hydrogen bonding involving the neighbouring amine and hydroxyl groups. As a result, 2-aminophenol has a relatively high melting point (174 °C) compared to other compounds with a similar molecular mass; for example, 2-m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2-Aminophenol
2-Aminophenol is an organic compound with the formula C6H7NO. Along with its isomer 4-aminophenol, it is an amphoteric molecule and a reducing agent. It is a useful reagent for the synthesis of dyes and heterocyclic compounds.Mitchell, S.C. & Waring, R.H. "Aminophenols." In Ullmann’s Encyclopedia of Industrial Chemistry; 2002 Wiley-VCH, . Reflecting its slight hydrophilic character, white powder is moderately soluble in alcohols and can be recrystallized from hot water. Synthesis and structure 2-Aminophenol (and its isomer, 4-aminophenol) is industrially synthesized by reducing the corresponding nitrophenol by hydrogen in the presence of various catalysts. The nitrophenols can also be reduced with iron. The compound exhibits intra- and intermolecular hydrogen bonding involving the neighbouring amine and hydroxyl groups. As a result, 2-aminophenol has a relatively high melting point (174 °C) compared to other compounds with a similar molecular mass; for example, 2-m ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aniline
Aniline is an organic compound with the formula C6 H5 NH2. Consisting of a phenyl group attached to an amino group, aniline is the simplest aromatic amine. It is an industrially significant commodity chemical, as well as a versatile starting material for fine chemical synthesis. Its main use is in the manufacture of precursors to polyurethane, dyes, and other industrial chemicals. Like most volatile amines, it has the odor of rotten fish. It ignites readily, burning with a smoky flame characteristic of aromatic compounds. It is toxic to humans. Relative to benzene, it is electron-rich. It thus participates more rapidly in electrophilic aromatic substitution reactions. Likewise, it is also prone to oxidation: while freshly purified aniline is an almost colorless oil, exposure to air results in gradual darkening to yellow or red, due to the formation of strongly colored, oxidized impurities. Aniline can be diazotized to give a diazonium salt, which can then undergo var ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rodinal
Rodinal is the trade name of a black and white developing agent produced originally by the German company Agfa based on the chemical 4-aminophenol 4-Aminophenol (or ''para''-aminophenol or ''p''-aminophenol) is an organic compound with the formula H2NC6H4OH. Typically available as a white powder, it is commonly used as a developer for black-and-white film, marketed under the name Rodinal. R .... See also * Film development References External links Ed Buffaloe's "Appreciating Rodinal" ArticleAn introduction to RodinalFilm developing with RodinalHistoric Rodinal Formulae{Photography-stub Photographic chemicals Agfa ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Royal Society Of Chemistry
The Royal Society of Chemistry (RSC) is a learned society (professional association) in the United Kingdom with the goal of "advancing the chemical sciences". It was formed in 1980 from the amalgamation of the Chemical Society, the Royal Institute of Chemistry, the Faraday Society, and the Society for Analytical Chemistry with a new Royal Charter and the dual role of learned society and professional body. At its inception, the Society had a combined membership of 34,000 in the UK and a further 8,000 abroad. The headquarters of the Society are at Burlington House, Piccadilly, London. It also has offices in Thomas Graham House in Cambridge (named after Thomas Graham, the first president of the Chemical Society) where ''RSC Publishing'' is based. The Society has offices in the United States, on the campuses of The University of Pennsylvania and Drexel University, at the University City Science Center in Philadelphia, Pennsylvania, in both Beijing and Shanghai, China and in Ba ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photographic Developer
In the processing of photographic films, plates or papers, the photographic developer (or just developer) is one or more chemicals that convert the latent image to a visible image. Developing agents achieve this conversion by reducing the silver halides, which are pale-colored, into silver metal, which is black (when a fine particle).Karlheinz Keller et al. ''Photography'' in ''Ullmann's Encyclopedia of Industrial Chemistry'', 2005, Wiley-VCH, Weinheim. The conversion occurs within the gelatine matrix. The special feature of photography is that the developer acts more quickly on those particles of silver halides that have been exposed to light. Paper left in developer will eventually reduce all the silver halides and turn black. Generally, the longer a developer is allowed to work, the darker the image. Chemical composition of developers The developer typically consists of a mixture of chemical compounds prepared as an aqueous solution. For black-and-white photography, three ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Nitration
In organic chemistry, nitration is a general class of chemical processes for the introduction of a nitro group into an organic compound. The term also is applied incorrectly to the different process of forming nitrate esters between alcohols and nitric acid (as occurs in the synthesis of nitroglycerin). The difference between the resulting molecular structures of nitro compounds and nitrates is that the nitrogen atom in nitro compounds is directly bonded to a non-oxygen atom (typically carbon or another nitrogen atom), whereas in nitrate esters (also called organic nitrates), the nitrogen is bonded to an oxygen atom that in turn usually is bonded to a carbon atom (nitrito group). There are many major industrial applications of nitration in the strict sense; the most important by volume are for the production of nitroaromatic compounds such as nitrobenzene. Nitration reactions are notably used for the production of explosives, for example the conversion of guanidine to nitrogua ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Phenol
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it requires careful handling because it can cause chemical burns. Phenol was first extracted from coal tar, but today is produced on a large scale (about 7 billion kg/year) from petroleum-derived feedstocks. It is an important industrial commodity as a precursor to many materials and useful compounds. It is primarily used to synthesize plastics and related materials. Phenol and its chemical derivatives are essential for production of polycarbonates, epoxies, Bakelite, nylon, detergents, herbicides such as phenoxy herbicides, and numerous pharmaceutical drugs. Properties Phenol is an organic compound appreciably soluble in water, with about 84.2 g dissolving in 1000 mL (0.895 M). Homogeneous mixtures of phenol and water ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formulae – that is, same number of atoms of each element – but distinct arrangements of atoms in space. Isomerism is existence or possibility of isomers. Isomers do not necessarily share similar chemical or physical properties. Two main forms of isomerism are structural or constitutional isomerism, in which ''bonds'' between the atoms differ; and stereoisomerism or spatial isomerism, in which the bonds are the same but the ''relative positions'' of the atoms differ. Isomeric relationships form a hierarchy. Two chemicals might be the same constitutional isomer, but upon deeper analysis be stereoisomers of each other. Two molecules that are the same stereoisomer as each other might be in different conformational forms or be different isotopologues. The depth of analysis depends on the field of study or the chemical and physical properties of interest. The English word "isomer" () is a back ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
N-methylaminophenol
Metol (or Elon) is a trade name for the organic compound with the formula OC6H4NH2(CH3)SO4. It is the sulfate salt of ''N''-methylaminophenol. This colourless salt is a popular photographic developer used in black & white photography.Gerd Löbbert "Photography" in Ullmann's Encyclopedia of Industrial Chemistry, 2005, Wiley-VCH, Weinheim. Synthesis Several methods exist for the preparation of ''N''-methylaminophenol. It arises by decarboxylation of ''N''-4-hydroxyphenylglycine ( Glycin). It can be obtained by reaction of hydroquinone with methylamine. Application Metol is an excellent developing agent for most continuous tone developer applications, and it has been widely used in published developer formulas as well as commercial products. However, it is difficult to produce highly concentrated developer solutions using Metol, and therefore most Metol developers are supplied in dry chemical mix. A developer containing both Metol and hydroquinone is called an MQ developer. Th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |