|
3,4-Ethylenedioxythiophene
3,4-Ethylenedioxythiophene (EDOT) is an organosulfur compound with the formula C2H4O2C4H2S. The molecule consists of thiophene, substituted at the 3 and 4 positions with an ethylene glycolyl unit. It is a colorless viscous liquid. EDOT is the precursor to the polymer PEDOT, which is found in electrochromism, electrochromic displays, photovoltaics, electroluminescent displays, printed wiring, and sensors. Synthesis and polymerization The original synthesis proceeded via the diester of 3,4-dihydroxythiophene-2,5-dicarboxylate. EDOT is often prepared from C4 precursors such as butanediol and butadiene via routes that produce the thiophene and dioxane rings in separate steps. Representative is the reaction of 2,3-butanedione, trimethyl orthoformate, and ethylene glycol to form the dioxane. Sulfidization with elemental sulfur gives the bicyclic target. EDOT is converted into the conducting polymer PEDOT by oxidation. The mechanism for this conversion begins with production of th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
PEDOT
Poly(3,4-ethylenedioxythiophene) (PEDOT or PEDT; ''IUPAC'' name poly(2,3-dihydrothieno[3,4-''b''][1,4]dioxane-5,7-diyl)) is a conducting polymer based on 3,4-Ethylenedioxythiophene, 3,4-ethylenedioxythiophene or EDOT. It was first reported by Bayer AG in 1989. Polymer PEDOT possesses many advantageous properties compared to earlier conducting polythiophenes like Polythiophene#3-Alkylthiophenes, 3-alkylthiophenes. For example, the polymer is Transparency (optics), optically transparent in its electrical conduction, conducting state and has high stability, moderate band gap, and low oxidation potential, redox potential. Its major disadvantage is its poor solubility, which is partly circumvented by use of composite materials such as PEDOT:PSS and PEDOT-TMA. The polymer is generated by oxidation. The process begins with production of the radical cation of EDOT monomer, [C2H4O2C4H2S]+. This cation adds to a neutral EDOT followed by deprotonation. The idealized conversion using peroxy ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
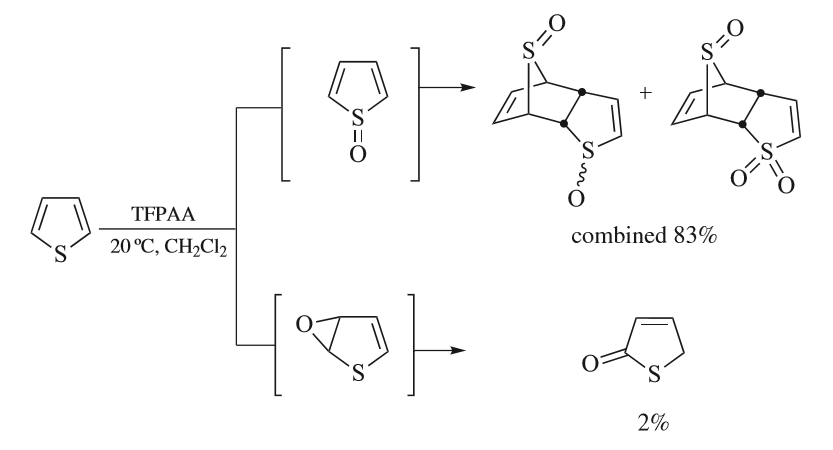
Thiophene
Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a planar five-membered ring, it is aromatic as indicated by its extensive substitution reactions. It is a colorless liquid with a benzene-like odor. In most of its reactions, it resembles benzene. Compounds analogous to thiophene include furan (C4H4O), selenophene (C4H4Se) and pyrrole (C4H4NH), which each vary by the heteroatom in the ring. Isolation and occurrence Thiophene was discovered as a contaminant in benzene. It was observed that isatin (an indole) forms a blue dye if it is mixed with sulfuric acid and crude benzene. The formation of the blue indophenin had long been believed to be a reaction of benzene itself. Viktor Meyer was able to isolate thiophene as the actual substance responsible for this reaction. Thiophene and especially its derivatives occur in petroleum, sometimes in concentrations up to 1–3%. The thiophenic content of oil and coal is removed via the hydrodesulfurization ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Thiophenes
Thiophene is a heterocyclic compound with the formula C4H4S. Consisting of a planar five-membered ring, it is aromatic as indicated by its extensive substitution reactions. It is a colorless liquid with a benzene-like odor. In most of its reactions, it resembles benzene. Compounds analogous to thiophene include furan (C4H4O), selenophene (C4H4Se) and pyrrole (C4H4NH), which each vary by the heteroatom in the ring. Isolation and occurrence Thiophene was discovered as a contaminant in benzene. It was observed that isatin (an indole) forms a blue dye if it is mixed with sulfuric acid and crude benzene. The formation of the blue indophenin had long been believed to be a reaction of benzene itself. Viktor Meyer was able to isolate thiophene as the actual substance responsible for this reaction. Thiophene and especially its derivatives occur in petroleum, sometimes in concentrations up to 1–3%. The thiophenic content of oil and coal is removed via the hydrodesulfurization (HDS) ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Organosulfur Compound
Organosulfur compounds are organic compounds that contain sulfur. They are often associated with foul odors, but many of the sweetest compounds known are organosulfur derivatives, e.g., saccharin. Nature abounds with organosulfur compounds—sulfur is vital for life. Of the 20 common amino acids, two ( cysteine and methionine) are organosulfur compounds, and the antibiotics penicillin and sulfa drugs both contain sulfur. While sulfur-containing antibiotics save many lives, sulfur mustard is a deadly chemical warfare agent. Fossil fuels, coal, petroleum, and natural gas, which are derived from ancient organisms, necessarily contain organosulfur compounds, the removal of which is a major focus of oil refineries. Sulfur shares the chalcogen group with oxygen, selenium, and tellurium, and it is expected that organosulfur compounds have similarities with carbon–oxygen, carbon–selenium, and carbon–tellurium compounds. A classical chemical test for the detection of sulfur ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Electrochromism
Electrochromism is a phenomenon in which a material displays changes in color or opacity in response to an electrical stimulus. In this way, a smart window made of an electrochromic material can block specific wavelengths of ultraviolet, visible or (near) infrared light. The ability to control transmittance of near infrared light can increase the energy efficiency of a building, reducing the amount of energy needed to cool during summer and heat during winter. As the color change is persistent and energy need only be applied to effect a change, electrochromic materials are used to control the amount of light and heat allowed to pass through a surface, most commonly "smart windows". One popular application is in the automobile industry where it is used to automatically tint rear-view mirrors in various lighting conditions. Principle The phenomenon of electrochromism occurs in some transition metal oxides which conduct both electricity and ions, such as tungsten trioxide ( ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Photovoltaic
Photovoltaics (PV) is the conversion of light into electricity using semiconducting materials that exhibit the photovoltaic effect, a phenomenon studied in physics, photochemistry, and electrochemistry. The photovoltaic effect is commercially used for electricity generation and as photosensors. A photovoltaic system employs solar modules, each comprising a number of solar cells, which generate electrical power. PV installations may be ground-mounted, rooftop-mounted, wall-mounted or floating. The mount may be fixed or use a solar tracker to follow the sun across the sky. Photovoltaic technology helps to mitigate climate change because it emits much less carbon dioxide than fossil fuels. Solar PV has specific advantages as an energy source: once installed, its operation generates no pollution and no greenhouse gas emissions, it shows scalability in respect of power needs and silicon has large availability in the Earth's crust, although other materials required in PV system ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Butanediol
Butanediol, also called butylene glycol, may refer to any one of four stable structural isomers: * 1,2-Butanediol * 1,3-Butanediol *1,4-Butanediol *2,3-Butanediol 2,3-Butanediol is the organic compound with the formula (CH3CHOH)2. It is classified as a ''vic''-diol ( glycol). It exists as three stereoisomers, a chiral pair and the meso isomer. All are colorless liquids. Applications include precursors ... Alkanediols {{Short pages monitor ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Butadiene
1,3-Butadiene () is the organic compound with the formula (CH2=CH)2. It is a colorless gas that is easily condensed to a liquid. It is important industrially as a precursor to synthetic rubber. The molecule can be viewed as the union of two vinyl groups. It is the simplest conjugated diene. Although butadiene breaks down quickly in the atmosphere, it is nevertheless found in ambient air in urban and suburban areas as a consequence of its constant emission from motor vehicles. The name butadiene can also refer to the isomer, 1,2-butadiene, which is a cumulated diene with structure H2C=C=CH−CH3. This allene has no industrial significance. History In 1863, the French chemist E. Caventou isolated butadiene from the pyrolysis of amyl alcohol. This hydrocarbon was identified as butadiene in 1886, after Henry Edward Armstrong isolated it from among the pyrolysis products of petroleum. In 1910, the Russian chemist Sergei Lebedev polymerized butadiene and obtained a materia ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Biacetyl
Diacetyl (IUPAC systematic name: butanedione or butane-2,3-dione) is an organic compound with the chemical formula (CH3CO)2. It is a yellow liquid with an intensely buttery flavor. It is a vicinal diketone (two C=O groups, side-by-side). Diacetyl occurs naturally in alcoholic beverages and is added as a flavoring to some foods to impart its buttery flavor. Chemical structure A distinctive feature of diacetyl (and other vicinal diketones) is the long C–C bond linking the carbonyl centers. This bond distance is about 1.54 Å, compared to 1.45 Å for the corresponding C–C bond in 1,3-butadiene. The elongation is attributed to repulsion between the polarized carbonyl carbon centers. Occurrence and biosynthesis Diacetyl arises naturally as a byproduct of fermentation. In some fermentative bacteria, it is formed via the thiamine pyrophosphate-mediated condensation of pyruvate and acetyl CoA. Sour (cultured) cream, cultured buttermilk, and cultured butter are produc ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Trimethyl Orthoformate
Trimethyl orthoformate (TMOF) is the organic compound with the formula HC(OCH3)3. A colorless liquid, it is the simplest orthoester. It is a reagent used in organic synthesis for the formation of methyl ethers. The product of reaction of an aldehyde with trimethyl orthoformate is an acetal. In general cases, these acetals can be deprotected back to the aldehyde by using hydrochloric acid. Synthesis Trimethyl orthoformate is prepared on an industrial scale by the methanolysis of hydrogen cyanide:Ashford's Dictionary of Industrial Chemicals, Third edition, 2011, , page 9388 :HCN + 3 HOCH3 → HC(OCH3)3 + NH3 Trimethyl orthoformate can also be prepared from the reaction between chloroform and sodium methoxide, an example of the Williamson ether synthesis. Use Trimethyl orthoformate is a useful building block for creating methoxymethylene groups and heterocyclic ring systems. It introduces a formyl group to a nucleophilic substrate, e.g. RNH2 to form R-NH-CHO, which can und ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Ethylene Glycol
Ethylene glycol (IUPAC name: ethane-1,2-diol) is an organic compound (a vicinal diol) with the formula . It is mainly used for two purposes, as a raw material in the manufacture of polyester fibers and for antifreeze formulations. It is an odorless, colorless, flammable, viscous liquid. Ethylene glycol has a sweet taste, but it is toxic in high concentrations. Production Industrial routes Ethylene glycol is produced from ethylene (ethene), via the intermediate ethylene oxide. Ethylene oxide reacts with water to produce ethylene glycol according to the chemical equation: This reaction can be catalyzed by either acids or bases, or can occur at neutral pH under elevated temperatures. The highest yields of ethylene glycol occur at acidic or neutral pH with a large excess of water. Under these conditions, ethylene glycol yields of 90% can be achieved. The major byproducts are the oligomers diethylene glycol, triethylene glycol, and tetraethylene glycol. The separation o ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |