|
2,6-Dimethoxybenzoquinone
2,6-Dimethoxybenzoquinone (2,6-DMBQ) is a chemical compound, classified as a benzoquinone, that has been found in ''Rauvolfia vomitoria'' and in '' Tibouchina pulchra''. Toxicity At physiological concentrations 2,6-dimethoxybenzoquinone is an antibacterial substance. At higher concentrations there is evidence that it is mutagenic,Brambilla G; Robbiano L; Cajelli E; Martelli A; Turmolini F; Mazzei M Cytotoxic, DNA-damaging and mutagenic properties of 2,6-dimethoxy-1,4-benzoquinone, formed by dimethophrine-nitrite interaction. The Journal of Pharmacology and Experimental Therapeutics (1988), 244(3), 1011-5 cytotoxic, genotoxic, and hepatotoxic Hepatotoxicity (from ''hepatic toxicity'') implies chemical-driven liver damage. Drug-induced liver injury is a cause of acute and chronic liver disease caused specifically by medications and the most common reason for a drug to be withdrawn fr .... Some reports have challenged its mutagenicity and others exclude such a possibility.In ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Tibouchina Pulchra
''Pleroma raddianum'', synonyms including ''Pleroma pulchrum'' (Cham.) Triana and ''Tibouchina pulchra'', is a plant species in the family Melastomataceae. It is a pioneer tree species, growing after land degradation, in the Atlantic Rainforest of Sao Paulo State in Brazil, a forest which only 12 percent of original area remains. 2,6-Dimethoxybenzoquinone 2,6-Dimethoxybenzoquinone (2,6-DMBQ) is a chemical compound, classified as a benzoquinone, that has been found in ''Rauvolfia vomitoria'' and in '' Tibouchina pulchra''. Toxicity At physiological concentrations 2,6-dimethoxybenzoquinone is an ant ... is a toxic chemical compound found in ''P. raddianum''. File:Tibouchina pulchra - Jardim Botânico de São Paulo - IMG 0269.jpg, Specimen in the Museu Botânico Dr. João Barbosa Rodrigues, Jardim Botânico de São Paulo, São Paulo City, Brazil File:Tibouchina pulchra - Jardim Botânico de São Paulo - IMG 0324.jpg, References External links raddianum ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Rauvolfia Vomitoria
''Rauvolfia vomitoria'', the poison devil's-pepper, is a plant species in the genus ''Rauvolfia''. It is native from Senegal east to Sudan and Tanzania, south to Angola; and naturalized in China, Bangladesh, different ranges of Himalayan and Puerto Rico. The plant contains a number of compounds of interest to the pharmaceutical industry and is widely used in traditional medicine. Description ''Rauvolfia vomitoria'' is a small tree or large shrub, growing to high. The branches grow in whorls, and the leaves grow from swollen nodes in groups of three. The leaf blades are broadly lanceolate or elliptical, tapering to a long point. The small, fragrant flowers are followed by globular red fruit. All parts of the plant, except the mature wood, contain latex. Ecology This is a fast-growing tree that produces large quantities of seeds which are dispersed by birds. The seedlings and saplings are tolerant of shade and the tree regenerates after cutting or burning, soon forming dense th ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
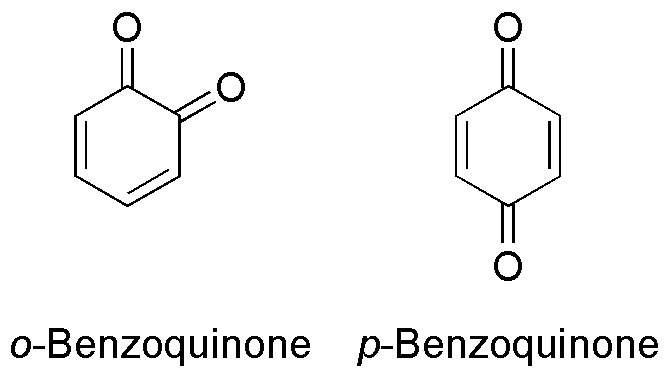
Benzoquinone
Benzoquinone (C6H4O2) is a quinone with a single benzene ring. There are 2 (out of 3 hypothetical) benzoquinones: * 1,4-Benzoquinone, most commonly, right image (also ''para''-benzoquinone, ''p''-benzoquinone, ''para''-quinone, or just quinone) * 1,2-Benzoquinone, less commonly, left image (also ''ortho''-benzoquinone, ''o''-benzoquinone, ''ortho''-quinone) *1,3-benzoquinone "does not exist, because its structure would be nonplanar and highly strained", though derivatives are known. An alkylated ''p''-benzoquinone has been found in the rhizomes of ''Iris kemaonensis''. See also * Arene substitution pattern Arene substitution patterns are part of organic chemistry IUPAC nomenclature and pinpoint the position of substituents other than hydrogen in relation to each other on an aromatic hydrocarbon. ''Ortho'', ''meta'', and ''para'' substitution ... References {{Chemistry index ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Mutagenic
In genetics, a mutagen is a physical or chemical agent that permanently changes genetic material, usually DNA, in an organism and thus increases the frequency of mutations above the natural background level. As many mutations can cause cancer in animals, such mutagens can therefore be carcinogens, although not all necessarily are. All mutagens have characteristic mutational signatures with some chemicals becoming mutagenic through cellular processes. The process of DNA becoming modified is called mutagenesis. Not all mutations are caused by mutagens: so-called "spontaneous mutations" occur due to spontaneous hydrolysis, errors in DNA replication, repair and recombination. Discovery The first mutagens to be identified were carcinogens, substances that were shown to be linked to cancer. Tumors were described more than 2,000 years before the discovery of chromosomes and DNA; in 500 B.C., the Greek physician Hippocrates named tumors resembling a crab ''karkinos'' (from which the ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Cytotoxic
Cytotoxicity is the quality of being toxic to cells. Examples of toxic agents are an immune cell or some types of venom, e.g. from the puff adder (''Bitis arietans'') or brown recluse spider (''Loxosceles reclusa''). Cell physiology Treating cells with the cytotoxic compound can result in a variety of cell fates. The cells may undergo necrosis, in which they lose membrane integrity and die rapidly as a result of cell lysis. The cells can stop actively growing and dividing (a decrease in cell viability), or the cells can activate a genetic program of controlled cell death (apoptosis). Cells undergoing necrosis typically exhibit rapid swelling, lose membrane integrity, shut down metabolism, and release their contents into the environment. Cells that undergo rapid necrosis in vitro do not have sufficient time or energy to activate apoptotic machinery and will not express apoptotic markers. Apoptosis is characterized by well defined cytological and molecular events including a cha ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Genotoxic
Genotoxicity is the property of chemical agents that damage the genetic information within a cell causing mutations, which may lead to cancer. While genotoxicity is often confused with mutagenicity, all mutagens are genotoxic, but some genotoxic substances are not mutagenic. The alteration can have direct or indirect effects on the DNA: the induction of mutations, mistimed event activation, and direct DNA damage leading to mutations. The permanent, heritable changes can affect either somatic cells of the organism or germ cells to be passed on to future generations. Cells prevent expression of the genotoxic mutation by either DNA repair or apoptosis; however, the damage may not always be fixed leading to mutagenesis. To assay for genotoxic molecules, researchers assay for DNA damage in cells exposed to the toxic substrates. This DNA damage can be in the form of single- and double-strand breaks, loss of excision repair, cross-linking, alkali-labile sites, point mutations, and st ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hepatotoxic
Hepatotoxicity (from ''hepatic toxicity'') implies chemical-driven liver damage. Drug-induced liver injury is a cause of acute and chronic liver disease caused specifically by medications and the most common reason for a drug to be withdrawn from the market after approval. The liver plays a central role in transforming and clearing chemicals and is susceptible to the toxicity from these agents. Certain medicinal agents, when taken in overdoses (e.g. paracetamol) and sometimes even when introduced within therapeutic ranges (e.g. halothane), may injure the organ. Other chemical agents, such as those used in laboratories and industries, natural chemicals (e.g., microcystins), and herbal remedies (two prominent examples being kava, mechanism unknown, and comfrey, through its pyrrolizidine alkaloid content) can also induce hepatotoxicity. Chemicals that cause liver injury are called hepatotoxins. More than 900 drugs have been implicated in causing liver injury (see LiverTox, exter ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Toxicological Sciences
''Toxicological Sciences'' is a monthly peer-reviewed scientific journal which covers all aspects of research on toxicology. It is published by Oxford University Press on behalf of the Society of Toxicology. It was established in 1981 as ''Fundamental and Applied Toxicology'' and obtained its current name in 1998. The current editor-in-chief is Jeffrey M. Peters, a professor of molecular toxicology and carcinogenesis at The Pennsylvania State University, and the Managing Editor is Virginia F. Hawkins. The editorial staff also includes Associate Editors in subject areas and an editorial board of topic experts. While its ISO 4 abbreviation is ''Toxicol. Sci.'' it is commonly referred to as ''ToxSci''. Abstracting and indexing The journal is abstracted and indexed in Biological Abstracts, BIOSIS, CAB International, Chemical Abstracts Service, Current Contents, EMBASE, Health & Safety Science Abstracts, Science Citation Index, and Toxicology Abstracts. According to the ''Jou ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |