|
17α-Epiestriol
17α-Epiestriol, or simply 17-epiestriol, also known as 16α-hydroxy-17α-estradiol or estra-1,3,5(10)-triene-3,16α,17α-triol, is a minor and weak endogenous estrogen, and the 17α-epimer of estriol (which is 16α-hydroxy-17β-estradiol). It is formed from 16α-hydroxyestrone. In contrast to other endogenous estrogens like estradiol, 17α-epiestriol is a selective agonist of the ERβ. It is described as a relatively weak estrogen, which is in accordance with its relatively low affinity for the ERα. 17α-Epiestriol has been found to be approximately 400-fold more potent than estradiol in inhibiting tumor necrosis factor α (TNFα)-induced vascular cell adhesion molecule 1 (VCAM-1) expression ''in vitro''. See also * Epimestrol * 16β,17α-Epiestriol * 16β-Epiestriol * 17α-Estradiol * 2-Methoxyestradiol 2-Methoxyestradiol (2-ME2, 2-MeO-E2) is a natural metabolite of estradiol and 2-hydroxyestradiol (2-OHE2). It is specifically the 2-methyl ether of 2-hydroxyestra ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
16β-Epiestriol
Epiestriol () (brand names Actriol, Arcagynil, Klimadoral), or epioestriol (), also known as 16β-epiestriol or simply 16-epiestriol as well as 16β-hydroxy-17β-estradiol, is a minor and weak endogenous estrogen (medication), estrogen, and the 16β-epimer of estriol (which is 16α-hydroxy-17β-estradiol). Epiestriol is (or has previously been) used clinically in the treatment of acne. In addition to its estrogenic actions, epiestriol has been found to possess significant anti-inflammatory properties without glycogenesis, glycogenic activity or immunosuppressive effects, an interesting finding that is in contrast to conventional anti-inflammatory steroids like hydrocortisone (a glucocorticoid). See also * 17α-Epiestriol * 16β,17α-Epiestriol * Epimestrol * Mytatrienediol References Anti-acne preparations Anti-inflammatory agents Estranes Estrogens Hormones of the hypothalamus-pituitary-gonad axis Sex hormones {{Genito-urinary-drug-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epiestriol
Epiestriol () (brand names Actriol, Arcagynil, Klimadoral), or epioestriol (), also known as 16β-epiestriol or simply 16-epiestriol as well as 16β-hydroxy-17β-estradiol, is a minor and weak endogenous estrogen, and the 16β-epimer of estriol (which is 16α-hydroxy-17β-estradiol). Epiestriol is (or has previously been) used clinically in the treatment of acne. In addition to its estrogenic actions, epiestriol has been found to possess significant anti-inflammatory properties without glycogenic activity or immunosuppressive effects, an interesting finding that is in contrast to conventional anti-inflammatory steroids like hydrocortisone (a glucocorticoid). See also * 17α-Epiestriol * 16β,17α-Epiestriol * Epimestrol * Mytatrienediol Mytatrienediol (developmental code name SC-6924; former tentative brand names Manvene, Anvene), also known as 16α-methyl-16β-epiestriol 3-methyl ether or 16β-hydroxy-16α-methylestradiol 3-methyl ether, is a synthetic steroidal estr ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
16β,17α-Epiestriol
16β,17α-Epiestriol, or 16,17-epiestriol, also known as 16β-hydroxy-17α-estradiol, as well as estra-1,3,5(10)-triene-3,16β,17α-triol, is a minor and weak endogenous steroidal estrogen that is related to 17α-estradiol and estriol. Along with estriol, 16β,17α-epiestriol has been detected in the urine of women during the late pregnancy stage. It shows preferential affinity for the ERβ over the ERα. See also * 16β-Epiestriol * 17α-Epiestriol 17α-Epiestriol, or simply 17-epiestriol, also known as 16α-hydroxy-17α-estradiol or estra-1,3,5(10)-triene-3,16α,17α-triol, is a minor and weak endogenous estrogen, and the 17α-epimer of estriol (which is 16α-hydroxy-17β-estradiol). It is ... * Epimestrol References {{DEFAULTSORT:Epiestriol, 16β,17α- Estranes Estrogens ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Endogenous
Endogenous substances and processes are those that originate from within a living system such as an organism, tissue, or cell. In contrast, exogenous substances and processes are those that originate from outside of an organism. For example, estradiol is an endogenous estrogen hormone produced within the body, whereas ethinylestradiol is an exogenous synthetic estrogen, commonly used in birth control pills Oral contraceptives, abbreviated OCPs, also known as birth control pills, are medications taken by mouth for the purpose of birth control. Female Two types of female oral contraceptive pill, taken once per day, are widely available: * The combi .... References External links *{{Wiktionary-inline, endogeny Biology ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Estrogens
Estrogen or oestrogen is a category of sex hormone responsible for the development and regulation of the female reproductive system and secondary sex characteristics. There are three major endogenous estrogens that have estrogenic hormonal activity: estrone (E1), estradiol (E2), and estriol (E3). Estradiol, an estrane, is the most potent and prevalent. Another estrogen called estetrol (E4) is produced only during pregnancy. Estrogens are synthesized in all vertebrates and some insects. Their presence in both vertebrates and insects suggests that estrogenic sex hormones have an ancient evolutionary history. Quantitatively, estrogens circulate at lower levels than androgens in both men and women. While estrogen levels are significantly lower in males than in females, estrogens nevertheless have important physiological roles in males. Like all steroid hormones, estrogens readily diffuse across the cell membrane. Once inside the cell, they bind to and activate estrogen recep ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Estranes
Estrane is a C18 steroid derivative, with a gonane core. ''Estrenes'' are estrane derivatives that contain a double bond, with an example being nandrolone. ''Estratrienes'' (estrins) are estrane derivatives that contain three double bonds, for instance estrin (estra-1,3,5(10)-triene). The estrogen steroid hormones estradiol Estradiol (E2), also spelled oestradiol, is an estrogen steroid hormone and the major female sex hormone. It is involved in the regulation of the estrous and menstrual female reproductive cycles. Estradiol is responsible for the development o ..., estrone, and estriol are estra-1,3,5(10)-trienes. See also * Androstane * Pregnane References Estranes {{Steroid-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
2-Methoxyestradiol
2-Methoxyestradiol (2-ME2, 2-MeO-E2) is a natural metabolite of estradiol and 2-hydroxyestradiol (2-OHE2). It is specifically the 2-methyl ether of 2-hydroxyestradiol. 2-Methoxyestradiol prevents the formation of new blood vessels that tumors need in order to grow (angiogenesis), hence it is an angiogenesis inhibitor. It also acts as a vasodilator and induces apoptosis in some cancer cell lines. 2-Methoxyestradiol is derived from estradiol, although it interacts poorly with the estrogen receptors (2,000-fold lower activational potency relative to estradiol). However, it retains activity as a high-affinity agonist of the G protein-coupled estrogen receptor (GPER) (10 nM, relative to 3–6 nM for estradiol). Clinical development 2-Methoxyestradiol was being developed as an experimental drug candidate with the tentative brand name Panzem. It has undergone Phase 1 clinical trials against breast cancer. A phase II trial of 18 advanced ovarian cancer patients reported en ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
17α-Estradiol
17α-Estradiol (also known as 17α-E2, 17-epiestradiol, alfatradiol, or estra-1,3,5(10)-triene-3,17α-diol) is a minor and weak endogenous steroidal estrogen that is related to 17β-estradiol (better known simply as estradiol). It is the C17 epimer of estradiol. It has approximately 100-fold lower estrogenic potency than 17β-estradiol. The compound shows preferential affinity for the ERα over the ERβ. Although 17α-estradiol is far weaker than 17β-estradiol as an agonist of the nuclear estrogen receptors, it has been found to bind to and activate the brain-expressed ER-X with a greater potency than that of 17β-estradiol, suggesting that it may be the predominant endogenous ligand for the receptor. Aging Supplementation with 17α-Estradiol increases the median lifespan of male mice by 19%, while not affecting female lifespan. This treatment does not lead to feminization of male mice. 17α-Estradiol furthermore alleviates age-related metabolic and inflammatory dysfu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Epimestrol
Epimestrol (, , ) (brand names Alene, Stimovul; former developmental code name ORG-817), also known as 3-methoxy-17-epiestriol, is a synthetic, steroidal estrogen and an estrogen ether and prodrug of 17-epiestriol. It has been used as a component of ovulation induction in combination with gonadotropin-releasing hormone. See also * List of estrogens This is a list of steroidal estrogens or derivatives of estradiol, estrone, and estriol. Most esters of these estrogens are not included in this list; for esters, see here instead. Estradiol derivatives 17α-Substituted estradiol derivative ... References Estranes Ethers Synthetic estrogens Triols {{Steroid-stub ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Hydrocortisone
Hydrocortisone is the name for the hormone cortisol when supplied as a medication. Uses include conditions such as adrenocortical insufficiency, adrenogenital syndrome, high blood calcium, thyroiditis, rheumatoid arthritis, dermatitis, asthma, and COPD. It is the treatment of choice for adrenocortical insufficiency. It can be given by mouth, topically, or by injection. Stopping treatment after long-term use should be done slowly. Side effects may include mood changes, increased risk of infection, and edema (swelling). With long-term use common side effects include osteoporosis, upset stomach, physical weakness, easy bruising, and candidiasis (yeast infections). While used, it is unclear if it is safe during pregnancy. Hydrocortisone is a glucocorticoid and works as an anti-inflammatory and by immune suppression. Hydrocortisone was patented in 1936 and approved for medical use in 1941. It is on the World Health Organization's List of Essential Medicines. It is availabl ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
Aldosterone
Aldosterone is the main mineralocorticoid steroid hormone produced by the zona glomerulosa of the adrenal cortex in the adrenal gland. It is essential for sodium conservation in the kidney, salivary glands, sweat glands, and colon. It plays a central role in the homeostatic regulation of blood pressure, plasma sodium (Na+), and potassium (K+) levels. It does so primarily by acting on the mineralocorticoid receptors in the distal tubules and collecting ducts of the nephron. It influences the reabsorption of sodium and excretion of potassium (from and into the tubular fluids, respectively) of the kidney, thereby indirectly influencing water retention or loss, blood pressure, and blood volume.Marieb Human Anatomy & Physiology 9th edition, chapter:16, page:629, question number:14 When dysregulated, aldosterone is pathogenic and contributes to the development and progression of cardiovascular and kidney disease. Aldosterone has exactly the opposite function of the atrial natriu ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |
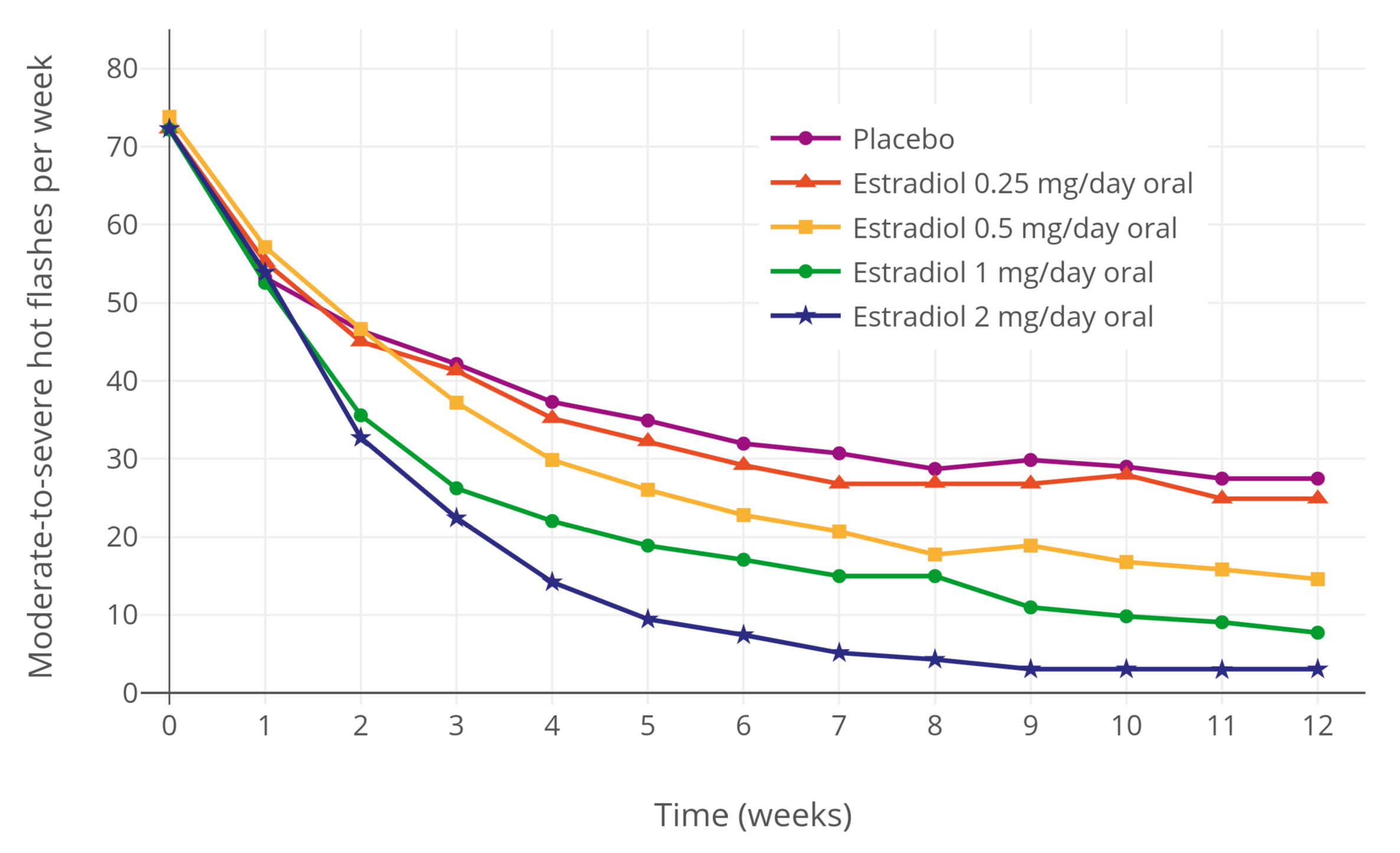
Estradiol (medication)
Estradiol (E2) is a medication and naturally occurring steroid hormone. It is an estrogen and is used mainly in menopausal hormone therapy and to treat low sex hormone levels in women. It is also used in hormonal birth control for women, in hormone therapy for transgender women, and in the treatment of hormone-sensitive cancers like prostate cancer in men and breast cancer in women, among other uses. Estradiol can be taken by mouth, held and dissolved under the tongue, as a gel or patch that is applied to the skin, in through the vagina, by injection into muscle or fat, or through the use of an implant that is placed into fat, among other routes. Side effects of estradiol in women include breast tenderness, breast enlargement, headache, fluid retention, and nausea among others. Men and children who are exposed to estradiol may develop symptoms of feminization, such as breast development and a feminine pattern of fat distribution, and men may also experience ... [...More Info...] [...Related Items...] OR: [Wikipedia] [Google] [Baidu] |