Yttrium on:
[Wikipedia]
[Google]
[Amazon]
 Yttrium is a
Yttrium is a
 Yttrium isotopes are among the most common products of the
Yttrium isotopes are among the most common products of the
 Johan Gadolin at the Royal Academy of Åbo (Turku) identified a new oxide (or "
Johan Gadolin at the Royal Academy of Åbo (Turku) identified a new oxide (or "
 Rare-earth elements (REEs) come mainly from four sources:
* Carbonate and fluoride containing ores such as the LREE bastnäsite ((Ce, La, etc.)(CO)F) contain on average 0.1% yttrium compared to the 99.9% for the 16 other REEs. The main source of bastnäsite from the 1960s to the 1990s was the Mountain Pass rare earth mine in California, making the United States the largest producer of REEs during that period. The name "bastnäsite" is actually a group name, and the Levinson suffix is used in the correct mineral names, e.g., bästnasite-(Y) has Y as a prevailing element.
* Monazite (( Ce, La, etc.) PO), which is mostly phosphate, is a
Rare-earth elements (REEs) come mainly from four sources:
* Carbonate and fluoride containing ores such as the LREE bastnäsite ((Ce, La, etc.)(CO)F) contain on average 0.1% yttrium compared to the 99.9% for the 16 other REEs. The main source of bastnäsite from the 1960s to the 1990s was the Mountain Pass rare earth mine in California, making the United States the largest producer of REEs during that period. The name "bastnäsite" is actually a group name, and the Levinson suffix is used in the correct mineral names, e.g., bästnasite-(Y) has Y as a prevailing element.
* Monazite (( Ce, La, etc.) PO), which is mostly phosphate, is a
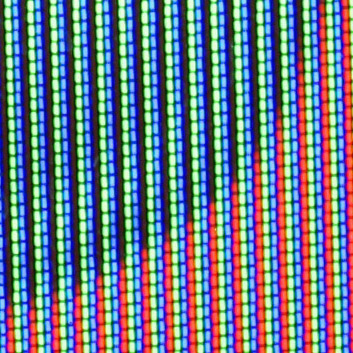 The red component of
The red component of
 Yttrium is used in the production of a large variety of synthetic garnets, and yttria is used to make yttrium iron garnets (, "YIG"), which are very effective
Yttrium is used in the production of a large variety of synthetic garnets, and yttria is used to make yttrium iron garnets (, "YIG"), which are very effective
 Yttrium is a key ingredient in the
Yttrium is a key ingredient in the
Yttrium by Paul C.W. Chu at acs.org
at ''
Encyclopedia of Geochemistry - Yttrium
{{Authority control Chemical elements Transition metals Deoxidizers Chemical elements with hexagonal close-packed structure
 Yttrium is a
Yttrium is a chemical element
A chemical element is a chemical substance whose atoms all have the same number of protons. The number of protons is called the atomic number of that element. For example, oxygen has an atomic number of 8: each oxygen atom has 8 protons in its ...
; it has symbol
A symbol is a mark, Sign (semiotics), sign, or word that indicates, signifies, or is understood as representing an idea, physical object, object, or wikt:relationship, relationship. Symbols allow people to go beyond what is known or seen by cr ...
Y and atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of its atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (''n''p) or the number of pro ...
39. It is a silvery-metallic transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinid ...
chemically similar to the lanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises at least the 14 metallic chemical elements with atomic numbers 57–70, from lanthanum through ytterbium. In the periodic table, they fill the 4f orbitals. Lutetium (el ...
s and has often been classified as a "rare-earth element
The rare-earth elements (REE), also called the rare-earth metals or rare earths, and sometimes the lanthanides or lanthanoids (although scandium and yttrium, which do not belong to this series, are usually included as rare earths), are a set o ...
". Yttrium is almost always found in combination with lanthanide elements in rare-earth mineral
A rare-earth mineral contains one or more rare-earth elements as major metal constituents. Rare-earth minerals are usually found in association with alkaline to peralkaline igneous magmas in pegmatites or with carbonatite Intrusive rock, intrusiv ...
s and is never found in nature as a free element. 89Y is the only stable isotope
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their Atomic nucleus, nuclei) and position in the periodic table (and hence belong to the same chemica ...
and the only isotope found in the Earth's crust
Earth's crust is its thick outer shell of rock, referring to less than one percent of the planet's radius and volume. It is the top component of the lithosphere, a solidified division of Earth's layers that includes the crust and the upper ...
.
The most important present-day use of yttrium is as a component of phosphor
A phosphor is a substance that exhibits the phenomenon of luminescence; it emits light when exposed to some type of radiant energy. The term is used both for fluorescent or phosphorescent substances which glow on exposure to ultraviolet or ...
s, especially those used in LED
A light-emitting diode (LED) is a semiconductor device that emits light when current flows through it. Electrons in the semiconductor recombine with electron holes, releasing energy in the form of photons. The color of the light (corresp ...
s. Historically, it was once widely used in the red phosphors in television set cathode ray tube
A cathode-ray tube (CRT) is a vacuum tube containing one or more electron guns, which emit electron beams that are manipulated to display images on a phosphorescent screen. The images may represent electrical waveforms on an oscilloscope, a ...
displays. Yttrium is also used in the production of electrode
An electrode is an electrical conductor used to make contact with a nonmetallic part of a circuit (e.g. a semiconductor, an electrolyte, a vacuum or a gas). In electrochemical cells, electrodes are essential parts that can consist of a varie ...
s, electrolyte
An electrolyte is a substance that conducts electricity through the movement of ions, but not through the movement of electrons. This includes most soluble Salt (chemistry), salts, acids, and Base (chemistry), bases, dissolved in a polar solven ...
s, electronic filters, laser
A laser is a device that emits light through a process of optical amplification based on the stimulated emission of electromagnetic radiation. The word ''laser'' originated as an acronym for light amplification by stimulated emission of radi ...
s, superconductors, various medical applications, and tracing various materials to enhance their properties.
Yttrium has no known biological
Biology is the scientific study of life and living organisms. It is a broad natural science that encompasses a wide range of fields and unifying principles that explain the structure, function, growth, origin, evolution, and distribution of ...
role. Exposure to yttrium compounds can cause lung disease
The lungs are the primary organs of the respiratory system in many animals, including humans. In mammals and most other tetrapods, two lungs are located near the backbone on either side of the heart. Their function in the respiratory syst ...
in humans.
Etymology
The element is named after '' ytterbite'', a mineral first identified in 1787 by the chemist Carl Axel Arrhenius. He named the mineral after the village ofYtterby
Ytterby () is a village on the Swedish island of Resarö, in Vaxholm Municipality in the Stockholm archipelago. Today the residential area is dominated by suburban homes.
The name of the village translates to "outer village". Ytterby is the ...
, in Sweden
Sweden, formally the Kingdom of Sweden, is a Nordic countries, Nordic country located on the Scandinavian Peninsula in Northern Europe. It borders Norway to the west and north, and Finland to the east. At , Sweden is the largest Nordic count ...
, where it had been discovered. When one of the chemicals in ytterbite was later found to be a previously unidentified element, the element was then named yttrium after the mineral.
Characteristics
Properties
Yttrium is a soft, silver-metallic, lustrous and highly crystallinetransition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinid ...
in group 3 Group 3 may refer to:
* Group 3 element, chemical element classification
* Group 3 (motorsport), FIA classification of cars used in auto racing and rallying
* Group 3, the third tier of races in worldwide Thoroughbred horse racing
* Group 3 image ...
. As expected by periodic trends, it is less electronegative
Electronegativity, symbolized as , is the tendency for an atom of a given chemical element to attract shared electrons (or electron density) when forming a chemical bond. An atom's electronegativity is affected by both its atomic number and the d ...
than its predecessor in the group, scandium
Scandium is a chemical element; it has Symbol (chemistry), symbol Sc and atomic number 21. It is a silvery-white metallic d-block, d-block element. Historically, it has been classified as a rare-earth element, together with yttrium and the lantha ...
, and less electronegative than the next member of period 5, zirconium
Zirconium is a chemical element; it has Symbol (chemistry), symbol Zr and atomic number 40. First identified in 1789, isolated in impure form in 1824, and manufactured at scale by 1925, pure zirconium is a lustrous transition metal with a greyis ...
. However, due to the lanthanide contraction
The lanthanide contraction is the greater-than-expected decrease in atomic radii and ionic radii of the elements in the lanthanide series, from left to right. It is caused by the poor shielding effect of nuclear charge by the 4f electrons alo ...
, it is also less electronegative than its successor in the group, lutetium
Lutetium is a chemical element; it has symbol Lu and atomic number 71. It is a silvery white metal, which resists corrosion in dry air, but not in moist air. Lutetium is the last element in the lanthanide series, and it is traditionally counted am ...
. Yttrium is the first d-block element in the fifth period.
The pure element is relatively stable in air in bulk form, due to passivation of a protective oxide () film that forms on the surface. This film can reach a thickness of 10 μm when yttrium is heated to 750 ° C in water vapor
Water vapor, water vapour, or aqueous vapor is the gaseous phase of Properties of water, water. It is one Phase (matter), state of water within the hydrosphere. Water vapor can be produced from the evaporation or boiling of liquid water or from th ...
. When finely divided, however, yttrium is very unstable in air; shavings or turnings of the metal can ignite in air at temperatures exceeding 400 °C. Yttrium nitride (YN) is formed when the metal is heated to 1000 °C in nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
.
Similarity to the lanthanides
The similarities of yttrium to thelanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises at least the 14 metallic chemical elements with atomic numbers 57–70, from lanthanum through ytterbium. In the periodic table, they fill the 4f orbitals. Lutetium (el ...
s are so strong that the element has been grouped with them as a rare-earth element
The rare-earth elements (REE), also called the rare-earth metals or rare earths, and sometimes the lanthanides or lanthanoids (although scandium and yttrium, which do not belong to this series, are usually included as rare earths), are a set o ...
, and is always found in nature together with them in rare-earth mineral
A rare-earth mineral contains one or more rare-earth elements as major metal constituents. Rare-earth minerals are usually found in association with alkaline to peralkaline igneous magmas in pegmatites or with carbonatite Intrusive rock, intrusiv ...
s. Emsley 2001, p. 498 Chemically, yttrium resembles those elements more closely than its neighbor in the periodic table, scandium
Scandium is a chemical element; it has Symbol (chemistry), symbol Sc and atomic number 21. It is a silvery-white metallic d-block, d-block element. Historically, it has been classified as a rare-earth element, together with yttrium and the lantha ...
, Daane 1968, p. 810. and if physical properties were plotted against atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of its atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (''n''p) or the number of pro ...
, it would have an apparent number of 64.5 to 67.5, placing it between the lanthanides gadolinium
Gadolinium is a chemical element; it has Symbol (chemistry), symbol Gd and atomic number 64. It is a silvery-white metal when oxidation is removed. Gadolinium is a malleable and ductile rare-earth element. It reacts with atmospheric oxygen or moi ...
and erbium
Erbium is a chemical element; it has Symbol (chemistry), symbol Er and atomic number 68. A silvery-white solid metal when artificially isolated, natural erbium is always found in chemical combination with other elements. It is a lanthanide, a rare- ...
. Daane 1968, p. 815.
It often also falls in the same range for reaction order, resembling terbium
Terbium is a chemical element; it has Symbol (chemistry), symbol Tb and atomic number 65. It is a silvery-white, rare earth element, rare earth metal that is malleable and ductile. The ninth member of the lanthanide series, terbium is a fairly ele ...
and dysprosium
Dysprosium is a chemical element; it has symbol Dy and atomic number 66. It is a rare-earth element in the lanthanide series with a metallic silver luster. Dysprosium is never found in nature as a free element, though, like other lanthanides, it ...
in its chemical reactivity. Yttrium is so close in size to the so-called 'yttrium group' of heavy lanthanide ions that in solution, it behaves as if it were one of them. Even though the lanthanides are one row farther down the periodic table than yttrium, the similarity in atomic radius may be attributed to the lanthanide contraction
The lanthanide contraction is the greater-than-expected decrease in atomic radii and ionic radii of the elements in the lanthanide series, from left to right. It is caused by the poor shielding effect of nuclear charge by the 4f electrons alo ...
.
One of the few notable differences between the chemistry of yttrium and that of the lanthanides is that yttrium is almost exclusively trivalent
In chemistry, the valence (US spelling) or valency (British spelling) of an atom is a measure of its combining capacity with other atoms when it forms chemical compounds or molecules. Valence is generally understood to be the number of chemica ...
, whereas about half the lanthanides can have valences other than three; nevertheless, only for four of the fifteen lanthanides are these other valences important in aqueous solution ( Ce, Sm, Eu, and Yb). Daane 1968, p. 817
Compounds and reactions
As a trivalent transition metal, yttrium forms variousinorganic compound
An inorganic compound is typically a chemical compound that lacks carbon–hydrogen bondsthat is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as ''inorganic chemistry''.
Inorgan ...
s, generally in the +3 oxidation state, by giving up all three of its valence electron
In chemistry and physics, valence electrons are electrons in the outermost shell of an atom, and that can participate in the formation of a chemical bond if the outermost shell is not closed. In a single covalent bond, a shared pair forms with b ...
s. A good example is yttrium(III) oxide (), also known as yttria, a six-coordinate
In geometry, a coordinate system is a system that uses one or more numbers, or coordinates, to uniquely determine and standardize the position of the points or other geometric elements on a manifold such as Euclidean space. The coordinates are ...
white solid.
Yttrium forms a water-insoluble fluoride
Fluoride (). According to this source, is a possible pronunciation in British English. is an Inorganic chemistry, inorganic, Monatomic ion, monatomic Ion#Anions and cations, anion of fluorine, with the chemical formula (also written ), whose ...
, hydroxide
Hydroxide is a diatomic anion with chemical formula OH−. It consists of an oxygen and hydrogen atom held together by a single covalent bond, and carries a negative electric charge. It is an important but usually minor constituent of water. It ...
, and oxalate
Oxalate (systematic IUPAC name: ethanedioate) is an anion with the chemical formula . This dianion is colorless. It occurs naturally, including in some foods. It forms a variety of salts, for example sodium oxalate (), and several esters such as ...
, but its bromide, chloride
The term chloride refers to a compound or molecule that contains either a chlorine anion (), which is a negatively charged chlorine atom, or a non-charged chlorine atom covalently bonded to the rest of the molecule by a single bond (). The pr ...
, iodide
An iodide ion is I−. Compounds with iodine in formal oxidation state −1 are called iodides. In everyday life, iodide is most commonly encountered as a component of iodized salt, which many governments mandate. Worldwide, iodine deficiency ...
, nitrate
Nitrate is a polyatomic ion with the chemical formula . salt (chemistry), Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives. Almost all inorganic nitrates are solubility, soluble in wa ...
and sulfate
The sulfate or sulphate ion is a polyatomic anion with the empirical formula . Salts, acid derivatives, and peroxides of sulfate are widely used in industry. Sulfates occur widely in everyday life. Sulfates are salts of sulfuric acid and many ...
are all soluble
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solubi ...
in water. The Y ion is colorless in solution due to the absence of electrons in the d and f electron shell
In chemistry and atomic physics, an electron shell may be thought of as an orbit that electrons follow around an atom's nucleus. The closest shell to the nucleus is called the "1 shell" (also called the "K shell"), followed by the "2 shell" (o ...
s.
Water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known liv ...
readily reacts with yttrium and its compounds to form . Concentrated nitric and hydrofluoric acid
Hydrofluoric acid is a solution of hydrogen fluoride (HF) in water. Solutions of HF are colorless, acidic and highly corrosive. A common concentration is 49% (48–52%) but there are also stronger solutions (e.g. 70%) and pure HF has a boiling p ...
s do not rapidly attack yttrium, but other strong acids do.
With halogen
The halogens () are a group in the periodic table consisting of six chemically related elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and the radioactive elements astatine (At) and tennessine (Ts), though some authors would ...
s, yttrium forms trihalides such as yttrium(III) fluoride (), yttrium(III) chloride (), and yttrium(III) bromide () at temperatures above roughly 200 °C. Similarly, carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
, phosphorus
Phosphorus is a chemical element; it has Chemical symbol, symbol P and atomic number 15. All elemental forms of phosphorus are highly Reactivity (chemistry), reactive and are therefore never found in nature. They can nevertheless be prepared ar ...
, selenium
Selenium is a chemical element; it has symbol (chemistry), symbol Se and atomic number 34. It has various physical appearances, including a brick-red powder, a vitreous black solid, and a grey metallic-looking form. It seldom occurs in this elem ...
, silicon
Silicon is a chemical element; it has symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic lustre, and is a tetravalent metalloid (sometimes considered a non-metal) and semiconductor. It is a membe ...
and sulfur
Sulfur ( American spelling and the preferred IUPAC name) or sulphur ( Commonwealth spelling) is a chemical element; it has symbol S and atomic number 16. It is abundant, multivalent and nonmetallic. Under normal conditions, sulfur atoms ...
all form binary compounds with yttrium at elevated temperatures.
Organoyttrium chemistry is the study of compounds containing carbon–yttrium bonds. A few of these are known to have yttrium in the oxidation state 0. (The +2 state has been observed in chloride melts, and +1 in oxide clusters in the gas phase.) Some trimerization reactions were generated with organoyttrium compounds as catalysts. These syntheses use as a starting material, obtained from and concentrated hydrochloric acid
Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungency, pungent smell. It is classified as a acid strength, strong acid. It is ...
and ammonium chloride
Ammonium chloride is an inorganic chemical compound with the chemical formula , also written as . It is an ammonium salt of hydrogen chloride. It consists of ammonium cations and chloride anions . It is a white crystalline salt (chemistry), sal ...
.
Hapticity
In coordination chemistry, hapticity is the coordination complex, coordination of a ligand to a metal center via an uninterrupted and contiguous series of atoms. The hapticity of a ligand is described with the Greek letter eta (letter), η ('eta ...
is a term to describe the coordination of a group of contiguous atoms of a ligand
In coordination chemistry, a ligand is an ion or molecule with a functional group that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's el ...
bound to the central atom; it is indicated by the Greek letter eta, η. Yttrium complexes were the first examples of complexes where carboranyl ligands were bound to a d-metal center through a η-hapticity. Vaporization of the graphite intercalation compounds graphite–Y or graphite– leads to the formation of endohedral fullerenes such as Y@C. Electron spin resonance
Electron paramagnetic resonance (EPR) or electron spin resonance (ESR) spectroscopy is a method for studying materials that have unpaired electrons. The basic concepts of EPR are analogous to those of nuclear magnetic resonance (NMR), but the spin ...
studies indicated the formation of Y and (C) ion pairs. The carbide
In chemistry, a carbide usually describes a compound composed of carbon and a metal. In metallurgy, carbiding or carburizing is the process for producing carbide coatings on a metal piece.
Interstitial / Metallic carbides
The carbides of th ...
s YC, YC, and YC can be hydrolyzed to form hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and Hydrophobe, hydrophobic; their odor is usually fain ...
s.
Isotopes and nucleosynthesis
Yttrium in theSolar System
The Solar SystemCapitalization of the name varies. The International Astronomical Union, the authoritative body regarding astronomical nomenclature, specifies capitalizing the names of all individual astronomical objects but uses mixed "Sola ...
was created by stellar nucleosynthesis
In astrophysics, stellar nucleosynthesis is the creation of chemical elements by nuclear fusion reactions within stars. Stellar nucleosynthesis has occurred since the original creation of hydrogen, helium and lithium during the Big Bang. As a ...
, mostly by the s-process
The slow neutron-capture process, or ''s''-process, is a series of nuclear reactions, reactions in nuclear astrophysics that occur in stars, particularly asymptotic giant branch stars. The ''s''-process is responsible for the creation (nucleosynt ...
(≈72%), but also the r-process
In nuclear astrophysics, the rapid neutron-capture process, also known as the ''r''-process, is a set of nuclear reactions that is responsible for nucleosynthesis, the creation of approximately half of the Atomic nucleus, atomic nuclei Heavy meta ...
(≈28%). The r-process consists of rapid neutron capture
Neutron capture is a nuclear reaction in which an atomic nucleus and one or more neutrons collide and merge to form a heavier nucleus. Since neutrons have no electric charge, they can enter a nucleus more easily than positively charged protons, wh ...
by lighter elements during supernova
A supernova (: supernovae or supernovas) is a powerful and luminous explosion of a star. A supernova occurs during the last stellar evolution, evolutionary stages of a massive star, or when a white dwarf is triggered into runaway nuclear fusion ...
explosions. The s-process is a slow neutron
The neutron is a subatomic particle, symbol or , that has no electric charge, and a mass slightly greater than that of a proton. The Discovery of the neutron, neutron was discovered by James Chadwick in 1932, leading to the discovery of nucle ...
capture of lighter elements inside pulsating red giant
A red giant is a luminous giant star of low or intermediate mass (roughly 0.3–8 solar masses ()) in a late phase of stellar evolution. The stellar atmosphere, outer atmosphere is inflated and tenuous, making the radius large and the surface t ...
stars.
 Yttrium isotopes are among the most common products of the
Yttrium isotopes are among the most common products of the nuclear fission
Nuclear fission is a reaction in which the nucleus of an atom splits into two or more smaller nuclei. The fission process often produces gamma photons, and releases a very large amount of energy even by the energetic standards of radioactiv ...
of uranium in nuclear explosions and nuclear reactors. In the context of nuclear waste
Radioactive waste is a type of hazardous waste that contains radioactive material. It is a result of many activities, including nuclear medicine, nuclear research, nuclear power generation, nuclear decommissioning, rare-earth mining, and nuclear ...
management, the most important isotopes of yttrium are Y and Y, with half-lives of 58.51 days and 64 hours, respectively. Though Y has a short half-life, it exists in secular equilibrium
In nuclear physics, secular equilibrium is a situation in which the quantity of a radioactive isotope remains constant because its production rate (e.g., due to decay of a parent isotope) is equal to its decay rate.
In radioactive decay
Secular e ...
with its long-lived parent isotope, strontium-90
Strontium-90 () is a radioactive isotope of strontium produced by nuclear fission, with a half-life of 28.79 years. It undergoes β− decay into yttrium-90, with a decay energy of 0.546 MeV. Strontium-90 has applications in medicine a ...
(Sr) (half-life 29 years).
All group 3 elements have an odd atomic number
The atomic number or nuclear charge number (symbol ''Z'') of a chemical element is the charge number of its atomic nucleus. For ordinary nuclei composed of protons and neutrons, this is equal to the proton number (''n''p) or the number of pro ...
, and therefore few stable isotope
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their Atomic nucleus, nuclei) and position in the periodic table (and hence belong to the same chemica ...
s. Scandium
Scandium is a chemical element; it has Symbol (chemistry), symbol Sc and atomic number 21. It is a silvery-white metallic d-block, d-block element. Historically, it has been classified as a rare-earth element, together with yttrium and the lantha ...
has one stable isotope
Stable nuclides are Isotope, isotopes of a chemical element whose Nucleon, nucleons are in a configuration that does not permit them the surplus energy required to produce a radioactive emission. The Atomic nucleus, nuclei of such isotopes are no ...
, and yttrium itself has only one stable isotope, Y, which is also the only isotope that occurs naturally. However, the lanthanide rare earths contain elements of even atomic number and many stable isotopes. Yttrium-89 is thought to be more abundant than it otherwise would be, due in part to the s-process, which allows enough time for isotopes created by other processes to decay by electron emission
In physics, electron emission is the ejection of an electron from the surface of matter, or, in beta decay (β− decay), where a beta particle (a fast energetic electron or positron) is emitted from an atomic nucleus transforming the original nuc ...
(neutron → proton).
Such a slow process tends to favor isotopes with atomic mass number
The mass number (symbol ''A'', from the German word: ''Atomgewicht'', "atomic weight"), also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus. It is approx ...
s (A = protons + neutrons) around 90, 138 and 208, which have unusually stable atomic nuclei
The atomic nucleus is the small, dense region consisting of protons and neutrons at the center of an atom, discovered in 1911 by Ernest Rutherford at the University of Manchester based on the 1909 Geiger–Marsden gold foil experiment. Aft ...
with 50, 82, and 126 neutrons, respectively. This stability is thought to result from their very low neutron-capture cross-section. Electron emission of isotopes with those mass numbers is simply less prevalent due to this stability, resulting in them having a higher abundance. 89Y has a mass number close to 90 and has 50 neutrons in its nucleus.
At least 32 synthetic isotopes of yttrium have been observed, and these range in atomic mass number
The mass number (symbol ''A'', from the German word: ''Atomgewicht'', "atomic weight"), also called atomic mass number or nucleon number, is the total number of protons and neutrons (together known as nucleons) in an atomic nucleus. It is approx ...
from 76 to 108. The least stable of these is Y with a half-life Half-life is a mathematical and scientific description of exponential or gradual decay.
Half-life, half life or halflife may also refer to:
Film
* Half-Life (film), ''Half-Life'' (film), a 2008 independent film by Jennifer Phang
* ''Half Life: ...
of 25 ms and the most stable is Y with half-life 106.629 days. Apart from Y, Y, and Y, with half-lives of 58.51 days, 79.8 hours, and 64 hours, respectively; all other isotopes have half-lives of less than a day and most of less than an hour.
Yttrium isotopes with mass numbers at or below 88 decay mainly by positron emission
Positron emission, beta plus decay, or β+ decay is a subtype of radioactive decay called beta decay, in which a proton inside a radionuclide nucleus is converted into a neutron while releasing a positron and an electron neutrino (). Positron emi ...
(proton → neutron) to form strontium
Strontium is a chemical element; it has symbol Sr and atomic number 38. An alkaline earth metal, it is a soft silver-white yellowish metallic element that is highly chemically reactive. The metal forms a dark oxide layer when it is exposed to ...
( Z = 38) isotopes. Yttrium isotopes with mass numbers at or above 90 decay mainly by electron emission (neutron → proton) to form zirconium
Zirconium is a chemical element; it has Symbol (chemistry), symbol Zr and atomic number 40. First identified in 1789, isolated in impure form in 1824, and manufactured at scale by 1925, pure zirconium is a lustrous transition metal with a greyis ...
(Z = 40) isotopes. Isotopes with mass numbers at or above 97 are also known to have minor decay paths of β delayed neutron emission
Neutron emission is a mode of radioactive decay in which one or more neutrons are ejected from a Atomic nucleus, nucleus. It occurs in the most neutron-rich/proton-deficient nuclides, and also from excited states of other nuclides as in photodisin ...
.
Yttrium has at least 20 metastable ("excited") isomers ranging in mass number from 78 to 102. Multiple excitation states have been observed for Y and Y. While most yttrium isomers are expected to be less stable than their ground state
The ground state of a quantum-mechanical system is its stationary state of lowest energy; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state ...
; Y have longer half-lives than their ground states, as these isomers decay by beta decay rather than isomeric transition
A nuclear isomer is a metastable state of an atomic nucleus, in which one or more nucleons (protons or neutrons) occupy excited state levels (higher energy levels). "Metastable" describes nuclei whose excited states have half-lives of 10−9 s ...
.
History
In 1787, part-time chemist Carl Axel Arrhenius found a heavy black rock in an old quarry near the Swedish village ofYtterby
Ytterby () is a village on the Swedish island of Resarö, in Vaxholm Municipality in the Stockholm archipelago. Today the residential area is dominated by suburban homes.
The name of the village translates to "outer village". Ytterby is the ...
(now part of the Stockholm Archipelago
The Stockholm Archipelago () is the largest archipelago in Sweden, and the second-largest archipelago in the Baltic Sea (the largest being the Archipelago Sea across the Baltic in Finland). Part of the archipelago has been designated as a Rams ...
). Thinking it was an unknown mineral containing the newly discovered element tungsten
Tungsten (also called wolfram) is a chemical element; it has symbol W and atomic number 74. It is a metal found naturally on Earth almost exclusively in compounds with other elements. It was identified as a distinct element in 1781 and first ...
, Emsley 2001, p. 496 he named it ''ytterbite'' and sent samples to various chemists for analysis. Van der Krogt 2005
 Johan Gadolin at the Royal Academy of Åbo (Turku) identified a new oxide (or "
Johan Gadolin at the Royal Academy of Åbo (Turku) identified a new oxide (or "earth
Earth is the third planet from the Sun and the only astronomical object known to Planetary habitability, harbor life. This is enabled by Earth being an ocean world, the only one in the Solar System sustaining liquid surface water. Almost all ...
") in Arrhenius' sample in 1789, and published his completed analysis in 1794. Anders Gustaf Ekeberg confirmed the identification in 1797 and named the new oxide ''yttria''. In the decades after Antoine Lavoisier
Antoine-Laurent de Lavoisier ( ; ; 26 August 17438 May 1794), When reduced without charcoal, it gave off an air which supported respiration and combustion in an enhanced way. He concluded that this was just a pure form of common air and that i ...
developed the first modern definition of chemical element
A chemical element is a chemical substance whose atoms all have the same number of protons. The number of protons is called the atomic number of that element. For example, oxygen has an atomic number of 8: each oxygen atom has 8 protons in its ...
s, it was believed that earths could be reduced to their elements, meaning that the discovery of a new earth was equivalent to the discovery of the element within, which in this case would have been ''yttrium''.
Friedrich Wöhler
Friedrich Wöhler Royal Society of London, FRS(For) HonFRSE (; 31 July 180023 September 1882) was a German chemist known for his work in both organic chemistry, organic and inorganic chemistry, being the first to isolate the chemical elements be ...
is credited with first isolating the metal in 1828 by reacting a volatile chloride that he believed to be yttrium chloride with potassium.
In 1843, Carl Gustaf Mosander found that samples of yttria contained three oxides: white yttrium oxide (yttria), yellow terbium oxide (confusingly, this was called 'erbia' at the time) and rose-colored erbium oxide (called 'terbia' at the time). A fourth oxide, ytterbium oxide, was isolated in 1878 by Jean Charles Galissard de Marignac
Jean Charles Galissard de Marignac (24 April 1817 – 15 April 1894) was a Swiss chemist whose work with atomic weights suggested the possibility of isotopes and the packing fraction of nuclei. His study of the rare earth elements led to ...
. New elements were later isolated from each of those oxides, and each element was named, in some fashion, after Ytterby, the village near the quarry where they were found (see ytterbium, terbium
Terbium is a chemical element; it has Symbol (chemistry), symbol Tb and atomic number 65. It is a silvery-white, rare earth element, rare earth metal that is malleable and ductile. The ninth member of the lanthanide series, terbium is a fairly ele ...
, and erbium
Erbium is a chemical element; it has Symbol (chemistry), symbol Er and atomic number 68. A silvery-white solid metal when artificially isolated, natural erbium is always found in chemical combination with other elements. It is a lanthanide, a rare- ...
). In the following decades, seven other new metals were discovered in "Gadolin's yttria". Since yttria was found to be a mineral and not an oxide, Martin Heinrich Klaproth
Martin Heinrich Klaproth (1 December 1743 – 1 January 1817) was a German chemist. He trained and worked for much of his life as an apothecary, moving in later life to the university. His shop became the second-largest apothecary in Berlin, and ...
renamed it gadolinite
Gadolinite, sometimes known as ytterbite, is a silicate mineral consisting principally of the silicates of cerium, lanthanum, neodymium, yttrium, beryllium, and iron with the formula . It is called gadolinite-(Ce) or gadolinite-(Y), depending o ...
in honor of Gadolin.
Until the early 1920s, the chemical symbol Yt was used for the element, after which Y came into common use.
In 1987, yttrium barium copper oxide
Yttrium barium copper oxide (YBCO) is a family of crystalline chemical compounds that display high-temperature superconductivity; it includes the first material ever discovered to become superconducting above the boiling point of liquid nitrogen ...
was found to achieve high-temperature superconductivity
High-temperature superconductivity (high-c or HTS) is superconductivity in materials with a critical temperature (the temperature below which the material behaves as a superconductor) above , the boiling point of liquid nitrogen. They are "high- ...
. It was only the second material known to exhibit this property, and it was the first-known material to achieve superconductivity
Superconductivity is a set of physical properties observed in superconductors: materials where Electrical resistance and conductance, electrical resistance vanishes and Magnetic field, magnetic fields are expelled from the material. Unlike an ord ...
above the (economically important) boiling point of nitrogen.
Occurrence
Abundance
Yttrium is found in mostrare-earth mineral
A rare-earth mineral contains one or more rare-earth elements as major metal constituents. Rare-earth minerals are usually found in association with alkaline to peralkaline igneous magmas in pegmatites or with carbonatite Intrusive rock, intrusiv ...
s, and some uranium ore
Uranium ore deposits are economically recoverable concentrations of uranium within Earth's crust. Uranium is one of the most common Chemical element, elements in Earth's crust, being 40 times more common than silver and 500 times more common than ...
s, but never in the Earth's crust as a free element. About 31 ppm of the Earth's crust is yttrium, making it the 43rd most abundant element. Yttrium is found in soil in concentrations between 10 and 150 ppm (dry weight average of 23 ppm) and in sea water at 9 ppt. Lunar rock samples collected during the American Apollo Project
The Apollo program, also known as Project Apollo, was the United States human spaceflight program led by NASA, which Moon landing, landed the first humans on the Moon in 1969. Apollo followed Project Mercury that put the first Americans in sp ...
have a relatively high content of yttrium. Stwertka 1998, p. 115.
Yttrium is not considered a "bone-seeker" like strontium
Strontium is a chemical element; it has symbol Sr and atomic number 38. An alkaline earth metal, it is a soft silver-white yellowish metallic element that is highly chemically reactive. The metal forms a dark oxide layer when it is exposed to ...
and lead
Lead () is a chemical element; it has Chemical symbol, symbol Pb (from Latin ) and atomic number 82. It is a Heavy metal (elements), heavy metal that is density, denser than most common materials. Lead is Mohs scale, soft and Ductility, malleabl ...
. Normally, as little as is found in the entire human body; human breast milk
Breast milk (sometimes spelled as breastmilk) or mother's milk is milk produced by the mammary glands in the breasts of women. Breast milk is the primary source of nutrition for newborn infants, comprising fats, proteins, carbohydrates, and a var ...
contains 4 ppm. Yttrium can be found in edible plants in concentrations between 20 ppm and 100 ppm (fresh weight), with cabbage
Cabbage, comprising several cultivars of '' Brassica oleracea'', is a leafy green, red (purple), or white (pale green) biennial plant grown as an annual vegetable crop for its dense-leaved heads. It is descended from the wild cabbage ( ''B.& ...
having the largest amount. With as much as 700 ppm, the seeds of woody plants have the highest known concentrations.
there are reports of the discovery of very large reserves of rare-earth elements in the deep seabed several hundred kilometers from the tiny Japanese island of Minami-Torishima Island, also known as Marcus Island. This location is described as having "tremendous potential" for rare-earth elements and yttrium (REY), according to a study published in ''Scientific Reports''. "This REY-rich mud has great potential as a rare-earth metal resource because of the enormous amount available and its advantageous mineralogical features," the study reads. The study shows that more than of rare-earth elements could be "exploited in the near future." As well as yttrium (Y), which is used in products like camera lenses and mobile phone screens, the rare-earth elements found are europium (Eu), terbium (Tb), and dysprosium (Dy).
Production
As yttrium is chemically similar to lanthanides, it occurs in the same ores (rare-earth mineral
A rare-earth mineral contains one or more rare-earth elements as major metal constituents. Rare-earth minerals are usually found in association with alkaline to peralkaline igneous magmas in pegmatites or with carbonatite Intrusive rock, intrusiv ...
s) and is extracted by the same refinement processes. A slight distinction is recognized between the light (LREE) and the heavy rare-earth elements (HREE), but the distinction is not perfect. Yttrium is concentrated in the HREE group due to its ion size, though it has a lower atomic mass
Atomic mass ( or ) is the mass of a single atom. The atomic mass mostly comes from the combined mass of the protons and neutrons in the nucleus, with minor contributions from the electrons and nuclear binding energy. The atomic mass of atoms, ...
.
 Rare-earth elements (REEs) come mainly from four sources:
* Carbonate and fluoride containing ores such as the LREE bastnäsite ((Ce, La, etc.)(CO)F) contain on average 0.1% yttrium compared to the 99.9% for the 16 other REEs. The main source of bastnäsite from the 1960s to the 1990s was the Mountain Pass rare earth mine in California, making the United States the largest producer of REEs during that period. The name "bastnäsite" is actually a group name, and the Levinson suffix is used in the correct mineral names, e.g., bästnasite-(Y) has Y as a prevailing element.
* Monazite (( Ce, La, etc.) PO), which is mostly phosphate, is a
Rare-earth elements (REEs) come mainly from four sources:
* Carbonate and fluoride containing ores such as the LREE bastnäsite ((Ce, La, etc.)(CO)F) contain on average 0.1% yttrium compared to the 99.9% for the 16 other REEs. The main source of bastnäsite from the 1960s to the 1990s was the Mountain Pass rare earth mine in California, making the United States the largest producer of REEs during that period. The name "bastnäsite" is actually a group name, and the Levinson suffix is used in the correct mineral names, e.g., bästnasite-(Y) has Y as a prevailing element.
* Monazite (( Ce, La, etc.) PO), which is mostly phosphate, is a placer deposit
In geology, a placer deposit or placer is an accumulation of valuable minerals formed by gravity separation from a specific source rock during sedimentary processes. The name is from the Spanish language, Spanish word ''placer'', meaning "alluviu ...
of sand created by the transportation and gravitational separation of eroded granite. Monazite as a LREE ore contains 2% (or 3%) Stwertka 1998, p. 116 yttrium. The largest deposits were found in India and Brazil in the early 20th century, making those two countries the largest producers of yttrium in the first half of that century. Of the monazite group, the Ce-dominant member, monazite-(Ce), is the most common one.
* Xenotime, a REE phosphate, is the main HREE ore containing as much as 60% yttrium as yttrium phosphate
Yttrium phosphate, YPO4, is the phosphate salt of yttrium. It occurs in nature as minerals xenotime and weinschenkite.minsocam.orgWEINSCHENKITE, YTTRIUM PHOSPHATE DIHYDRATE retrieved 16 May 2014
Preparation
Yttrium phosphate can be obtained ...
(YPO). This applies to xenotime-(Y). The largest mine is the Bayan Obo deposit in China, making China the largest exporter for HREE since the closure of the Mountain Pass mine in the 1990s.
* Ion absorption clays or Longnan clays are the weathering products of granite and contain only 1% of REEs. The final ore concentrate can contain as much as 8% yttrium. Ion absorption clays are mostly in southern China. Yttrium is also found in samarskite and fergusonite (which also stand for group names). Emsley 2001, p. 497
One method for obtaining pure yttrium from the mixed oxide ores is to dissolve the oxide in sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
and fractionate it by ion exchange
Ion exchange is a reversible interchange of one species of ion present in an insoluble solid with another of like charge present in a solution surrounding the solid. Ion exchange is used in softening or demineralizing of water, purification of ch ...
chromatography
In chemical analysis, chromatography is a laboratory technique for the Separation process, separation of a mixture into its components. The mixture is dissolved in a fluid solvent (gas or liquid) called the ''mobile phase'', which carries it ...
. With the addition of oxalic acid
Oxalic acid is an organic acid with the systematic name ethanedioic acid and chemical formula , also written as or or . It is the simplest dicarboxylic acid. It is a white crystalline solid that forms a colorless solution in water. Its name i ...
, the yttrium oxalate precipitates. The oxalate is converted into the oxide by heating under oxygen. By reacting the resulting yttrium oxide with hydrogen fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluori ...
, yttrium fluoride is obtained. When quaternary ammonium salts are used as extractants, most yttrium will remain in the aqueous phase. When the counter-ion is nitrate, the light lanthanides are removed, and when the counter-ion is thiocyanate, the heavy lanthanides are removed. In this way, yttrium salts of 99.999% purity are obtained. In the usual situation, where yttrium is in a mixture that is two-thirds heavy-lanthanide, yttrium should be removed as soon as possible to facilitate the separation of the remaining elements.
Annual world production of yttrium oxide had reached by 2001; by 2014 it had increased to . Global reserves of yttrium oxide were estimated in 2014 to be more than . The leading countries for these reserves included Australia, Brazil, China, India, and the United States. Only a few tonnes of yttrium metal are produced each year by reducing yttrium fluoride to a metal sponge with calcium
Calcium is a chemical element; it has symbol Ca and atomic number 20. As an alkaline earth metal, calcium is a reactive metal that forms a dark oxide-nitride layer when exposed to air. Its physical and chemical properties are most similar to it ...
magnesium
Magnesium is a chemical element; it has Symbol (chemistry), symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 ...
alloy. The temperature of an arc furnace, in excess of 1,600 °C, is sufficient to melt the yttrium.
Applications
Consumer
 The red component of
The red component of color television
Color television (American English) or colour television (British English) is a television transmission technology that also includes color information for the picture, so the video image can be displayed in color on the television set. It improv ...
cathode ray tube
A cathode-ray tube (CRT) is a vacuum tube containing one or more electron guns, which emit electron beams that are manipulated to display images on a phosphorescent screen. The images may represent electrical waveforms on an oscilloscope, a ...
s is typically emitted from an yttria () or yttrium oxide sulfide () host lattice doped with europium
Europium is a chemical element; it has symbol Eu and atomic number 63. It is a silvery-white metal of the lanthanide series that reacts readily with air to form a dark oxide coating. Europium is the most chemically reactive, least dense, and soft ...
(III) cation (Eu) phosphor
A phosphor is a substance that exhibits the phenomenon of luminescence; it emits light when exposed to some type of radiant energy. The term is used both for fluorescent or phosphorescent substances which glow on exposure to ultraviolet or ...
s. The red color itself is emitted from the europium while the yttrium collects energy from the electron gun
file:Egun.jpg, Electron gun from a cathode-ray tube
file:Vidicon Electron Gun.jpg, The electron gun from an RCA Vidicon video camera tube
An electron gun (also called electron emitter) is an electrical component in some vacuum tubes that produc ...
and passes it to the phosphor. Daane 1968, p. 818 Yttrium compounds can serve as host lattices for doping with different lanthanide
The lanthanide () or lanthanoid () series of chemical elements comprises at least the 14 metallic chemical elements with atomic numbers 57–70, from lanthanum through ytterbium. In the periodic table, they fill the 4f orbitals. Lutetium (el ...
cations. Tb can be used as a doping agent to produce green luminescence
Luminescence is a spontaneous emission of radiation from an electronically or vibrationally excited species not in thermal equilibrium with its environment. A luminescent object emits ''cold light'' in contrast to incandescence, where an obje ...
. As such yttrium compounds such as yttrium aluminium garnet (YAG) are useful for phosphors and are an important component of white LED
A light-emitting diode (LED) is a semiconductor device that emits light when current flows through it. Electrons in the semiconductor recombine with electron holes, releasing energy in the form of photons. The color of the light (corresp ...
s.
Yttria is used as a sintering
Sintering or frittage is the process of compacting and forming a solid mass of material by pressure or heat without melting it to the point of liquefaction. Sintering happens as part of a manufacturing process used with metals, ceramics, plas ...
additive in the production of porous silicon nitride.
Yttrium compounds are used as a catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
for ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon–carbon bond, carbon–carbon doub ...
polymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many fo ...
. As a metal, yttrium is used on the electrodes of some high-performance spark plugs. Yttrium is used in gas mantles for propane
Propane () is a three-carbon chain alkane with the molecular formula . It is a gas at standard temperature and pressure, but becomes liquid when compressed for transportation and storage. A by-product of natural gas processing and petroleum ref ...
lantern
A lantern is a source of lighting, often portable. It typically features a protective enclosure for the light sourcehistorically usually a candle, a oil lamp, wick in oil, or a thermoluminescence, thermoluminescent Gas mantle, mesh, and often a ...
s as a replacement for thorium
Thorium is a chemical element; it has symbol Th and atomic number 90. Thorium is a weakly radioactive light silver metal which tarnishes olive grey when it is exposed to air, forming thorium dioxide; it is moderately soft, malleable, and ha ...
, which is radioactive
Radioactive decay (also known as nuclear decay, radioactivity, radioactive disintegration, or nuclear disintegration) is the process by which an unstable atomic nucleus loses energy by radiation. A material containing unstable nuclei is conside ...
.
Garnets
 Yttrium is used in the production of a large variety of synthetic garnets, and yttria is used to make yttrium iron garnets (, "YIG"), which are very effective
Yttrium is used in the production of a large variety of synthetic garnets, and yttria is used to make yttrium iron garnets (, "YIG"), which are very effective microwave
Microwave is a form of electromagnetic radiation with wavelengths shorter than other radio waves but longer than infrared waves. Its wavelength ranges from about one meter to one millimeter, corresponding to frequency, frequencies between 300&n ...
filters
Filtration is a physical process that separates solid matter and fluid from a mixture.
Filter, filtering, filters or filtration may also refer to:
Science and technology
Computing
* Filter (higher-order function), in functional programming
* Fil ...
which were recently shown to have magnetic interactions more complex and longer-ranged than understood over the previous four decades. Yttrium, iron
Iron is a chemical element; it has symbol Fe () and atomic number 26. It is a metal that belongs to the first transition series and group 8 of the periodic table. It is, by mass, the most common element on Earth, forming much of Earth's o ...
, aluminium
Aluminium (or aluminum in North American English) is a chemical element; it has chemical symbol, symbol Al and atomic number 13. It has a density lower than that of other common metals, about one-third that of steel. Aluminium has ...
, and gadolinium
Gadolinium is a chemical element; it has Symbol (chemistry), symbol Gd and atomic number 64. It is a silvery-white metal when oxidation is removed. Gadolinium is a malleable and ductile rare-earth element. It reacts with atmospheric oxygen or moi ...
garnets (e.g. and ) have important magnetic
Magnetism is the class of physical attributes that occur through a magnetic field, which allows objects to attract or repel each other. Because both electric currents and magnetic moments of elementary particles give rise to a magnetic field, m ...
properties. YIG is also very efficient as an acoustic energy transmitter and transducer. Yttrium aluminium garnet
Yttrium aluminium garnet (YAG, Yttrium, Y3Aluminium, Al5Oxygen, O12) is a synthetic crystalline material of the garnet group. It is a Crystal system, cubic yttrium aluminium oxide phase, with other examples being YAlO3 (YAP) in a Crystal system, ...
( or YAG) has a hardness
In materials science, hardness (antonym: softness) is a measure of the resistance to plastic deformation, such as an indentation (over an area) or a scratch (linear), induced mechanically either by Pressing (metalworking), pressing or abrasion ...
of 8.5 and is also used as a gemstone
A gemstone (also called a fine gem, jewel, precious stone, semiprecious stone, or simply gem) is a piece of mineral crystal which, when cut or polished, is used to make jewellery, jewelry or other adornments. Certain Rock (geology), rocks (such ...
in jewelry (simulated diamond
Diamond is a Allotropes of carbon, solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. Diamond is tasteless, odourless, strong, brittle solid, colourless in pure form, a poor conductor of e ...
). Cerium
Cerium is a chemical element; it has Chemical symbol, symbol Ce and atomic number 58. It is a hardness, soft, ductile, and silvery-white metal that tarnishes when exposed to air. Cerium is the second element in the lanthanide series, and while it ...
-doped yttrium aluminium garnet (YAG:Ce) crystals are used as phosphors to make white LED
A light-emitting diode (LED) is a semiconductor device that emits light when current flows through it. Electrons in the semiconductor recombine with electron holes, releasing energy in the form of photons. The color of the light (corresp ...
s.
YAG, yttria, yttrium lithium fluoride (LiYF), and yttrium orthovanadate (YVO) are used in combination with dopant
A dopant (also called a doping agent) is a small amount of a substance added to a material to alter its physical properties, such as electrical or optics, optical properties. The amount of dopant is typically very low compared to the material b ...
s such as neodymium
Neodymium is a chemical element; it has Symbol (chemistry), symbol Nd and atomic number 60. It is the fourth member of the lanthanide series and is considered to be one of the rare-earth element, rare-earth metals. It is a hard (physics), hard, sli ...
, erbium
Erbium is a chemical element; it has Symbol (chemistry), symbol Er and atomic number 68. A silvery-white solid metal when artificially isolated, natural erbium is always found in chemical combination with other elements. It is a lanthanide, a rare- ...
, ytterbium in near-infrared
Infrared (IR; sometimes called infrared light) is electromagnetic radiation (EMR) with wavelengths longer than that of visible light but shorter than microwaves. The infrared spectral band begins with the waves that are just longer than those ...
laser
A laser is a device that emits light through a process of optical amplification based on the stimulated emission of electromagnetic radiation. The word ''laser'' originated as an acronym for light amplification by stimulated emission of radi ...
s. YAG lasers can operate at high power and are used for drilling and cutting metal. The single crystals of doped YAG are normally produced by the Czochralski process
The Czochralski method, also Czochralski technique or Czochralski process, is a method of crystal growth used to obtain single crystals (monocrystals) of semiconductors (e.g. silicon, germanium and gallium arsenide), metals (e.g. palladium, plati ...
.
Material enhancer
Small amounts of yttrium (0.1 to 0.2%) have been used to reduce the grain sizes ofchromium
Chromium is a chemical element; it has Symbol (chemistry), symbol Cr and atomic number 24. It is the first element in Group 6 element, group 6. It is a steely-grey, Luster (mineralogy), lustrous, hard, and brittle transition metal.
Chromium ...
, molybdenum
Molybdenum is a chemical element; it has Symbol (chemistry), symbol Mo (from Neo-Latin ''molybdaenum'') and atomic number 42. The name derived from Ancient Greek ', meaning lead, since its ores were confused with lead ores. Molybdenum minerals hav ...
, titanium
Titanium is a chemical element; it has symbol Ti and atomic number 22. Found in nature only as an oxide, it can be reduced to produce a lustrous transition metal with a silver color, low density, and high strength, resistant to corrosion in ...
, and zirconium
Zirconium is a chemical element; it has Symbol (chemistry), symbol Zr and atomic number 40. First identified in 1789, isolated in impure form in 1824, and manufactured at scale by 1925, pure zirconium is a lustrous transition metal with a greyis ...
. Yttrium is used to increase the strength
Strength may refer to:
Personal trait
*Physical strength, as in people or animals
*Character strengths like those listed in the Values in Action Inventory
*The exercise of willpower
Physics
* Mechanical strength, the ability to withstand ...
of aluminium and magnesium
Magnesium is a chemical element; it has Symbol (chemistry), symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 ...
alloys. The addition of yttrium to alloys generally improves workability, adds resistance to high-temperature recrystallization, and significantly enhances resistance to high-temperature oxidation
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is ...
(see graphite nodule discussion below).
Yttrium can be used to deoxidize vanadium
Vanadium is a chemical element; it has Symbol (chemistry), symbol V and atomic number 23. It is a hard, silvery-grey, malleable transition metal. The elemental metal is rarely found in nature, but once isolated artificially, the formation of an ...
and other non-ferrous metal
In metallurgy, non-ferrous metals are metals or alloys that do not contain iron ( allotropes of iron, ferrite, and so on) in appreciable amounts.
Generally more costly than ferrous metals, non-ferrous metals are used because of desirable pro ...
s. Yttria stabilizes the cubic form of zirconia in jewelry.
Yttrium has been studied as a nodulizer in ductile cast iron, forming the graphite
Graphite () is a Crystallinity, crystalline allotrope (form) of the element carbon. It consists of many stacked Layered materials, layers of graphene, typically in excess of hundreds of layers. Graphite occurs naturally and is the most stable ...
into compact nodules instead of flakes to increase ductility
Ductility refers to the ability of a material to sustain significant plastic Deformation (engineering), deformation before fracture. Plastic deformation is the permanent distortion of a material under applied stress, as opposed to elastic def ...
and fatigue resistance. Having a high melting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state of matter, state from solid to liquid. At the melting point the solid and liquid phase (matter), phase exist in Thermodynamic equilib ...
, yttrium oxide is used in some ceramic
A ceramic is any of the various hard, brittle, heat-resistant, and corrosion-resistant materials made by shaping and then firing an inorganic, nonmetallic material, such as clay, at a high temperature. Common examples are earthenware, porcela ...
and glass
Glass is an amorphous (non-crystalline solid, non-crystalline) solid. Because it is often transparency and translucency, transparent and chemically inert, glass has found widespread practical, technological, and decorative use in window pane ...
to impart shock resistance and low thermal expansion
Thermal expansion is the tendency of matter to increase in length, area, or volume, changing its size and density, in response to an increase in temperature (usually excluding phase transitions).
Substances usually contract with decreasing temp ...
properties. Those same properties make such glass useful in camera lens
A camera lens, photographic lens or photographic objective is an optical lens (optics), lens or assembly of lenses (compound lens) used in conjunction with a camera body and mechanism to Imaging, make images of objects either on photographic film ...
es.
Medical
The radioisotopeyttrium-90
Yttrium-90 () is a radioactive isotope of yttrium. Yttrium-90 has found a wide range of uses in radiation therapy to treat some forms of cancer
Cancer is a group of diseases involving Cell growth#Disorders, abnormal cell growth with the ...
(Y) is used to label drugs such as edotreotide and ibritumomab tiuxetan for the treatment of various cancer
Cancer is a group of diseases involving Cell growth#Disorders, abnormal cell growth with the potential to Invasion (cancer), invade or Metastasis, spread to other parts of the body. These contrast with benign tumors, which do not spread. Po ...
s, including lymphoma
Lymphoma is a group of blood and lymph tumors that develop from lymphocytes (a type of white blood cell). The name typically refers to just the cancerous versions rather than all such tumours. Signs and symptoms may include enlarged lymph node ...
, leukemia
Leukemia ( also spelled leukaemia; pronounced ) is a group of blood cancers that usually begin in the bone marrow and produce high numbers of abnormal blood cells. These blood cells are not fully developed and are called ''blasts'' or '' ...
, liver, ovarian, colorectal, pancreatic and bone cancers. Emsley 2001, p. 495 It works by adhering to monoclonal antibodies
A monoclonal antibody (mAb, more rarely called moAb) is an antibody produced from a Lineage (evolution), cell lineage made by cloning a unique white blood cell. All subsequent antibodies derived this way trace back to a unique parent cell.
Mon ...
, which in turn bind to cancer cells and kill them via intense β-radiation from the Y (see monoclonal antibody therapy
Monoclonal antibodies (mAbs) have varied therapeutic uses. It is possible to create a mAb that binds specifically to almost any extracellular target, such as cell surface proteins and cytokines. They can be used to render their target ineffectiv ...
).
A technique called radioembolization
Selective internal radiation therapy (SIRT), also known as transarterial radioembolization (TARE), radioembolization or intra-arterial microbrachytherapy is a form of radionuclide therapy used in interventional radiology to treat cancer. It is gene ...
is used to treat hepatocellular carcinoma
Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer in adults and is currently the most common cause of death in people with cirrhosis. HCC is the third leading cause of cancer-related deaths worldwide.
HCC most common ...
and liver metastasis
A liver metastasis is a malignant tumor in the liver that has spread from another organ that is affected by cancer. This can also be called secondary liver cancer or metastatic liver disease. The liver is a common site for metastatic disease becaus ...
. Radioembolization is a low toxicity, targeted liver cancer therapy that uses millions of tiny beads made of glass or resin containing Y. The radioactive microspheres are delivered directly to the blood vessels feeding specific liver tumors/segments or lobes. It is minimally invasive and patients can usually be discharged after a few hours. This procedure may not eliminate all tumors throughout the entire liver, but works on one segment or one lobe at a time and may require multiple procedures.
Also see radioembolization in the case of combined cirrhosis and hepatocellular carcinoma.
Needles made of Y, which can cut more precisely than scalpels, have been used to sever pain-transmitting nerve
A nerve is an enclosed, cable-like bundle of nerve fibers (called axons). Nerves have historically been considered the basic units of the peripheral nervous system. A nerve provides a common pathway for the Electrochemistry, electrochemical nerv ...
s in the spinal cord
The spinal cord is a long, thin, tubular structure made up of nervous tissue that extends from the medulla oblongata in the lower brainstem to the lumbar region of the vertebral column (backbone) of vertebrate animals. The center of the spinal c ...
, and Y is also used to carry out radionuclide synovectomy in the treatment of inflamed joints, especially knees, in people with conditions such as rheumatoid arthritis
Rheumatoid arthritis (RA) is a long-term autoimmune disorder that primarily affects synovial joint, joints. It typically results in warm, swollen, and painful joints. Pain and stiffness often worsen following rest. Most commonly, the wrist and h ...
.
A neodymium-doped yttrium–aluminium–garnet laser has been used in an experimental, robot-assisted radical prostatectomy
Prostatectomy (from the Ancient Greek language, Greek , "prostate" and , "excision") is the surgical removal of all or part of the prostate gland. This operation is done for benignity, benign conditions that cause urinary retention, as well as ...
in canines in an attempt to reduce collateral nerve and tissue damage, and erbium-doped lasers are coming into use for cosmetic skin resurfacing.
Superconductors
 Yttrium is a key ingredient in the
Yttrium is a key ingredient in the yttrium barium copper oxide
Yttrium barium copper oxide (YBCO) is a family of crystalline chemical compounds that display high-temperature superconductivity; it includes the first material ever discovered to become superconducting above the boiling point of liquid nitrogen ...
(YBaCuO, aka 'YBCO' or '1-2-3') superconductor developed at the University of Alabama in Huntsville and the University of Houston
The University of Houston (; ) is a Public university, public research university in Houston, Texas, United States. It was established in 1927 as Houston Junior College, a coeducational institution and one of multiple junior colleges formed in ...
in 1987. This superconductor is notable because the operating superconductivity temperature is above liquid nitrogen
Liquid nitrogen (LN2) is nitrogen in a liquid state at cryogenics, low temperature. Liquid nitrogen has a boiling point of about . It is produced industrially by fractional distillation of liquid air. It is a colorless, mobile liquid whose vis ...
's boiling point (77.1 K). Since liquid nitrogen is less expensive than the liquid helium
Liquid helium is a physical state of helium at very low temperatures at standard atmospheric pressures. Liquid helium may show superfluidity.
At standard pressure, the chemical element helium exists in a liquid form only at the extremely low temp ...
required for metallic superconductors, the operating costs for applications would be less.
The actual superconducting material is often written as YBa2Cu3O7–''d'', where ''d'' must be less than 0.7 for superconductivity. The reason for this is still not clear, but it is known that the vacancies occur only in certain places in the crystal, the copper oxide planes, and chains, giving rise to a peculiar oxidation state of the copper atoms, which somehow leads to the superconducting behavior.
The theory of low temperature superconductivity has been well understood since the BCS theory
In physics, the Bardeen–Cooper–Schrieffer (BCS) theory (named after John Bardeen, Leon Cooper, and John Robert Schrieffer) is the first microscopic theory of superconductivity since Heike Kamerlingh Onnes's 1911 discovery. The theory descr ...
of 1957. It is based on a peculiarity of the interaction between two electrons in a crystal lattice. However, the BCS theory does not explain high temperature superconductivity, and its precise mechanism is still a mystery. What is known is that the composition of the copper-oxide materials must be precisely controlled for superconductivity to occur.
This superconductor is a black and green, multi-crystal, multi-phase mineral. Researchers are studying a class of materials known as perovskite
Perovskite (pronunciation: ) is a calcium titanium oxide mineral composed of calcium titanate (chemical formula ). Its name is also applied to the class of compounds which have the same type of crystal structure as , known as the perovskite (stru ...
s that are alternative combinations of these elements, hoping to develop a practical high-temperature superconductor
High-temperature superconductivity (high-c or HTS) is superconductivity in materials with a critical temperature (the temperature below which the material behaves as a superconductor) above , the boiling point of liquid nitrogen. They are "high- ...
.
Lithium batteries
Yttrium is used in small quantities in the cathodes of someLithium iron phosphate battery
The lithium iron phosphate battery ( battery) or LFP battery (''lithium ferrophosphate'') is a type of lithium-ion battery using lithium iron phosphate () as the cathode material, and a graphitic carbon electrode with a metallic backing as t ...
(LFP), which are then commonly called LiFeYPO chemistry, or LYP. Similar to LFP, LYP batteries offer high energy density
In physics, energy density is the quotient between the amount of energy stored in a given system or contained in a given region of space and the volume of the system or region considered. Often only the ''useful'' or extractable energy is measure ...
, good safety and long life. But LYP offers higher cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device such as a lead-acid battery. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. Conventional curren ...
stability, and prolongs the life of the battery, by protecting the physical structure of the cathode
A cathode is the electrode from which a conventional current leaves a polarized electrical device such as a lead-acid battery. This definition can be recalled by using the mnemonic ''CCD'' for ''Cathode Current Departs''. Conventional curren ...
, especially at higher temperatures and higher charging / discharge current. LYP batteries find use in stationary applications ( off-grid solar systems), electric vehicles
An electric vehicle (EV) is a motor vehicle whose propulsion is powered fully or mostly by electricity. EVs encompass a wide range of transportation modes, including road vehicle, road and rail vehicles, electric boats and Submersible, submer ...
(some cars), as well other applications (submarines, ships), similar to LFP batteries, but often at improved safety and cycle life time. LYP cells have essentially the same nominal voltage as LFP, 3.25V, but the maximum charging voltage is 4.0V, and the charging and discharge characteristics are very similar.
Other applications
In 2009, Professor Mas Subramanian and associates atOregon State University
Oregon State University (OSU) is a Public university, public Land-grant university, land-grant research university in Corvallis, Oregon, United States. OSU offers more than 200 undergraduate degree programs and a variety of graduate and doctor ...
discovered that yttrium can be combined with indium
Indium is a chemical element; it has Symbol (chemistry), symbol In and atomic number 49. It is a silvery-white post-transition metal and one of the softest elements. Chemically, indium is similar to gallium and thallium, and its properties are la ...
and manganese
Manganese is a chemical element; it has Symbol (chemistry), symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese was first isolated in the 1770s. It is a transition m ...
to form an intensely blue
Blue is one of the three primary colours in the RYB color model, RYB colour model (traditional colour theory), as well as in the RGB color model, RGB (additive) colour model. It lies between Violet (color), violet and cyan on the optical spe ...
, non-toxic, inert, fade-resistant pigment
A pigment is a powder used to add or alter color or change visual appearance. Pigments are completely or nearly solubility, insoluble and reactivity (chemistry), chemically unreactive in water or another medium; in contrast, dyes are colored sub ...
, YInMn blue, the first new blue pigment discovered in 200 years.
Precautions
Yttrium can be highlytoxic
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a subst ...
to humans, animals and plants.
Water-soluble compounds of yttrium are considered mildly toxic, while its insoluble compounds are non-toxic. In experiments on animals, yttrium and its compounds caused lung and liver damage, though toxicity varies with different yttrium compounds. In rats, inhalation of yttrium citrate caused pulmonary edema
Pulmonary edema (British English: oedema), also known as pulmonary congestion, is excessive fluid accumulation in the tissue or air spaces (usually alveoli) of the lungs. This leads to impaired gas exchange, most often leading to shortness ...
and dyspnea
Shortness of breath (SOB), known as dyspnea (in AmE) or dyspnoea (in BrE), is an uncomfortable feeling of not being able to breathe well enough. The American Thoracic Society defines it as "a subjective experience of breathing discomfort that ...
, while inhalation of yttrium chloride caused liver edema, pleural effusion
A pleural effusion is accumulation of excessive fluid in the pleural space, the potential space that surrounds each lung.
Under normal conditions, pleural fluid is secreted by the parietal pleural capillaries at a rate of 0.6 millilitre per kilog ...
s, and pulmonary hyperemia. (public domain text)
Exposure to yttrium compounds in humans may cause lung disease. Workers exposed to airborne yttrium europium vanadate dust experienced mild eye, skin, and upper respiratory tract irritation—though this may be caused by the vanadium
Vanadium is a chemical element; it has Symbol (chemistry), symbol V and atomic number 23. It is a hard, silvery-grey, malleable transition metal. The elemental metal is rarely found in nature, but once isolated artificially, the formation of an ...
content rather than the yttrium. Acute exposure to yttrium compounds can cause shortness of breath, coughing, chest pain, and cyanosis
Cyanosis is the change of Tissue (biology), tissue color to a bluish-purple hue, as a result of decrease in the amount of oxygen bound to the hemoglobin in the red blood cells of the capillary bed. Cyanosis is apparent usually in the Tissue (bi ...
. The Occupational Safety and Health Administration
The Occupational Safety and Health Administration (OSHA; ) is a regulatory agency of the United States Department of Labor that originally had federal visitorial powers to inspect and examine workplaces. The United States Congress established ...
(OSHA) limits
Limit or Limits may refer to:
Arts and media
* ''Limit'' (manga), a manga by Keiko Suenobu
* ''Limit'' (film), a South Korean film
* Limit (music), a way to characterize harmony
* "Limit" (song), a 2016 single by Luna Sea
* "Limits", a 2009 ...
exposure to yttrium in the workplace to over an 8-hour workday. The National Institute for Occupational Safety and Health
The National Institute for Occupational Safety and Health (NIOSH, ) is the List of United States federal agencies, United States federal agency responsible for conducting research and making recommendations for the prevention of work-related occ ...
(NIOSH) recommended exposure limit (REL) is over an 8-hour workday. At levels of , yttrium is immediately dangerous to life and health. Yttrium dust is highly flammable.
See also
*Notes
References
Bibliography
* * * * * * *Further reading
* *External links
Yttrium by Paul C.W. Chu at acs.org
at ''
The Periodic Table of Videos
''Periodic Videos'' (also known as ''The Periodic Table of Videos'') is a video project and YouTube channel on chemistry. It consists of a series of videos about chemical elements and the periodic table, with additional videos on other topics i ...
'' (University of Nottingham)
*
Encyclopedia of Geochemistry - Yttrium
{{Authority control Chemical elements Transition metals Deoxidizers Chemical elements with hexagonal close-packed structure