Violet Phosphorus on:
[Wikipedia]
[Google]
[Amazon]

 Elemental
Elemental
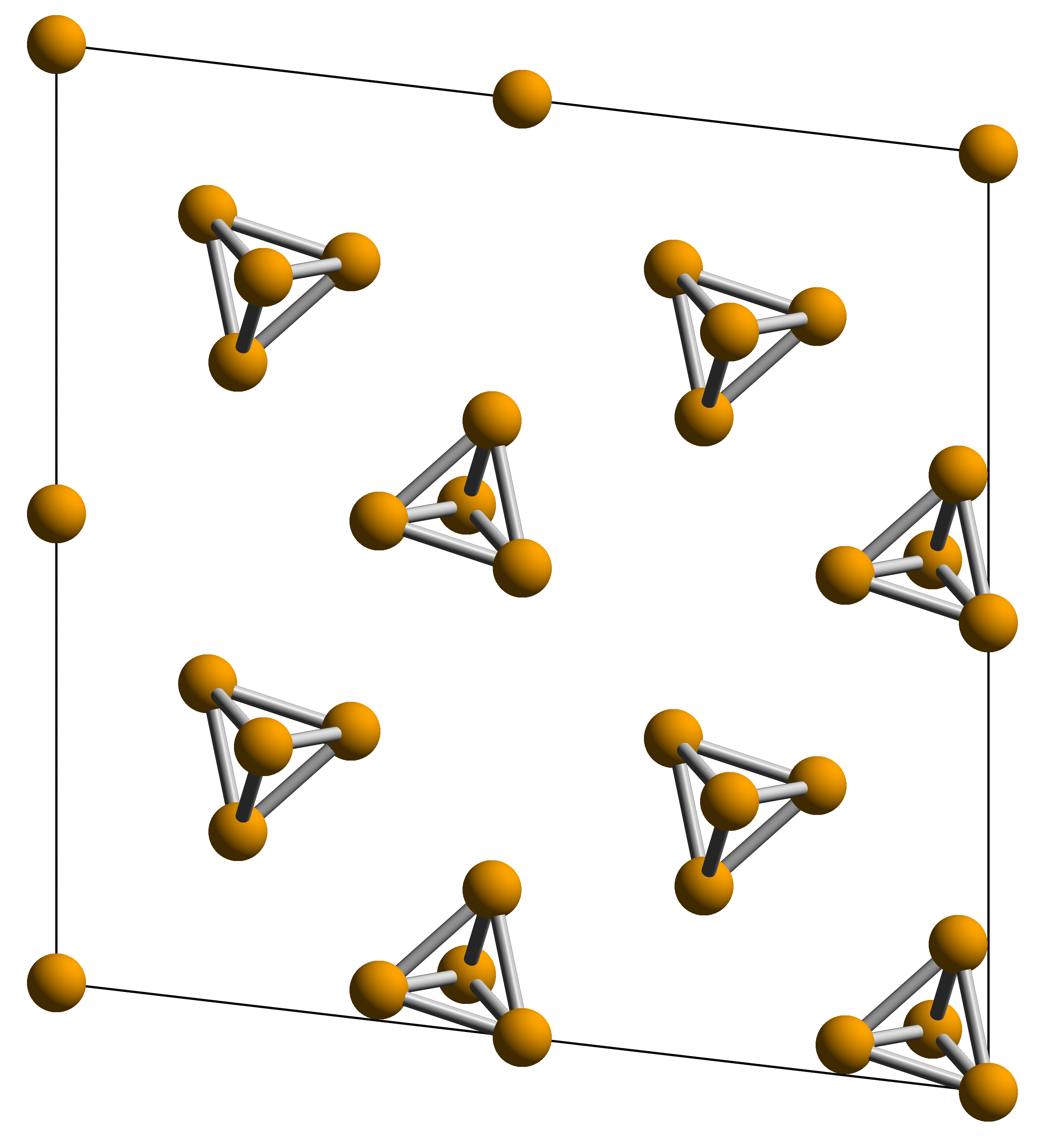 White phosphorus, yellow phosphorus or simply tetraphosphorus () exists as
White phosphorus, yellow phosphorus or simply tetraphosphorus () exists as
 Red phosphorus may be formed by heating
Red phosphorus may be formed by heating

 Monoclinic phosphorus, violet phosphorus, or Hittorf's metallic phosphorus is a crystalline form of the amorphous
Monoclinic phosphorus, violet phosphorus, or Hittorf's metallic phosphorus is a crystalline form of the amorphous
CSD-1935087
. The optical band gap of the violet phosphorus was measured by diffuse reflectance spectroscopy to be around 1.7 eV. The thermal decomposition temperature was 52 °C higher than its black phosphorus counterpart. The violet phosphorene was easily obtained from both mechanical and solution exfoliation.

 Black phosphorus is the thermodynamically stable form of phosphorus at room temperature and pressure, with a heat of formation of −39.3 kJ/mol (relative to white phosphorus which is defined as the standard state). It was first synthesized by heating white phosphorus under high pressures (12,000 atmospheres) in 1914. As a 2D material, in appearance, properties, and structure, black phosphorus is very much like
Black phosphorus is the thermodynamically stable form of phosphorus at room temperature and pressure, with a heat of formation of −39.3 kJ/mol (relative to white phosphorus which is defined as the standard state). It was first synthesized by heating white phosphorus under high pressures (12,000 atmospheres) in 1914. As a 2D material, in appearance, properties, and structure, black phosphorus is very much like

 The diphosphorus allotrope () can normally be obtained only under extreme conditions (for example, from at 1100 kelvin). In 2006, the diatomic molecule was generated in homogeneous solution under normal conditions with the use of
The diphosphorus allotrope () can normally be obtained only under extreme conditions (for example, from at 1100 kelvin). In 2006, the diatomic molecule was generated in homogeneous solution under normal conditions with the use of
White Phophorus
at ''
More about White Phosphorus (and phosphorus pentoxide)
at ''
The Chemistry of Phosphorus
at Chemistry LibreTexts.

phosphorus
Phosphorus is a chemical element; it has Chemical symbol, symbol P and atomic number 15. All elemental forms of phosphorus are highly Reactivity (chemistry), reactive and are therefore never found in nature. They can nevertheless be prepared ar ...
can exist in several allotropes
Allotropy or allotropism () is the property of some chemical elements to exist in two or more different forms, in the same physical state, known as allotropes of the elements. Allotropes are different structural modifications of an element: th ...
, the most common of which are white
White is the lightest color and is achromatic (having no chroma). It is the color of objects such as snow, chalk, and milk, and is the opposite of black. White objects fully (or almost fully) reflect and scatter all the visible wa ...
and red solids. Solid violet and black allotropes are also known. Gaseous phosphorus exists as diphosphorus and atomic phosphorus.
White phosphorus
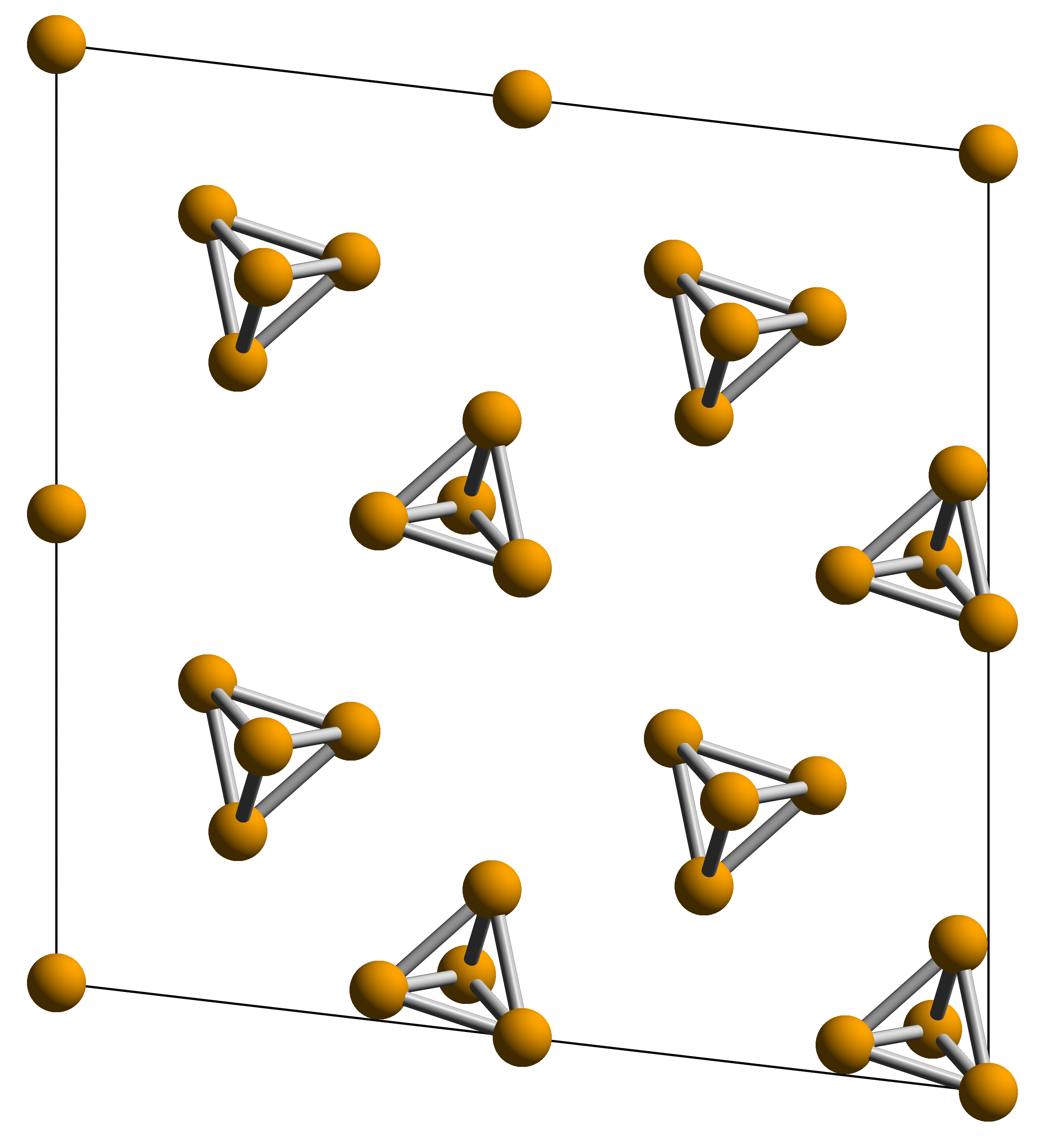 White phosphorus, yellow phosphorus or simply tetraphosphorus () exists as
White phosphorus, yellow phosphorus or simply tetraphosphorus () exists as molecules
A molecule is a group of two or more atoms that are held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemistry ...
of four phosphorus atoms
Atoms are the basic particles of the chemical elements. An atom consists of a nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished from each other ...
in a tetrahedral structure, joined by six phosphorus—phosphorus single bonds. The free P4 molecule in the gas phase has a P-P bond length of ''r''g = 2.1994(3) Å as was determined by gas electron diffraction. Despite the tetrahedral
In geometry, a tetrahedron (: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular Face (geometry), faces, six straight Edge (geometry), edges, and four vertex (geometry), vertices. The tet ...
arrangement the P4 molecules have no significant ring strain
In organic chemistry, ring strain is a type of instability that exists when bonds in a molecule form angles that are abnormal. Strain is most commonly discussed for small rings such as cyclopropanes and cyclobutanes, whose internal angles ar ...
and a vapor of P4 molecules is stable. This is due to the nature of bonding in the P4 tetrahedron which can be described by spherical aromaticity or cluster bonding, that is the electrons are highly delocalized. This has been illustrated by calculations of the magnetically induced currents, which sum up to 29 nA/T, much more than in the archetypical aromatic
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated system, conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugati ...
molecule benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
(11 nA/T).
Molten and gaseous white phosphorus also retains the tetrahedral molecules, until when it starts decomposing to molecules.
White phosphorus is a translucent waxy solid that quickly yellows in light, and impure white phosphorus is for this reason called yellow phosphorus. It is toxic
Toxicity is the degree to which a chemical substance or a particular mixture of substances can damage an organism. Toxicity can refer to the effect on a whole organism, such as an animal, bacterium, or plant, as well as the effect on a subst ...
, causing severe liver damage on ingestion and phossy jaw from chronic ingestion or inhalation.
It glows greenish in the dark (when exposed to oxygen). It ignites spontaneously in air at about , and at much lower temperatures if finely divided (due to melting-point depression). Because of this property, white phosphorus is used as a weapon. Phosphorus reacts with oxygen, usually forming ''two'' oxides depending on the amount of available oxygen: ( phosphorus trioxide) when reacted with a limited supply of oxygen, and when reacted with excess oxygen. On rare occasions, , , and are also formed, but in small amounts. This combustion gives phosphorus(V) oxide, which consists of tetrahedral with oxygen inserted between the phosphorus atoms and at their vertices:
:
The odour of combustion of this form has a characteristic garlic smell. White phosphorus is only slightly soluble in water and can be stored under water. Indeed, white phosphorus is safe from self-igniting when it is submerged in water; due to this, unreacted white phosphorus can prove hazardous to beachcombers who may collect washed-up samples while unaware of their true nature. is soluble in benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
, oils
An oil is any nonpolar chemical substance that is composed primarily of hydrocarbons and is hydrophobic (does not mix with water) and lipophilic (mixes with other oils). Oils are usually flammable and surface active. Most oils are unsaturat ...
, carbon disulfide, and disulfur dichloride
Disulfur dichloride (or disulphur dichloride by the British English spelling) is the inorganic compound of sulfur and chlorine with the Chemical formula, formula . It is an amber oily liquid.
Sometimes, this compound is incorrectly named ''sulfur ...
.
The white allotrope can be produced using several methods. In the industrial process, phosphate rock
Phosphorite, phosphate rock or rock phosphate is a non- detrital sedimentary rock that contains high amounts of phosphate minerals. The phosphate content of phosphorite (or grade of phosphate rock) varies greatly, from 4% to 20% phosphorus pentox ...
is heated in an electric or fuel-fired furnace in the presence of carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
and silica
Silicon dioxide, also known as silica, is an oxide of silicon with the chemical formula , commonly found in nature as quartz. In many parts of the world, silica is the major constituent of sand. Silica is one of the most complex and abundant f ...
.Threlfall, R.E., (1951). ''100 years of Phosphorus Making: 1851–1951''. Oldbury: Albright and Wilson Ltd Elemental phosphorus is then liberated as a vapour and can be collected under phosphoric acid
Phosphoric acid (orthophosphoric acid, monophosphoric acid or phosphoric(V) acid) is a colorless, odorless phosphorus-containing solid, and inorganic compound with the chemical formula . It is commonly encountered as an 85% aqueous solution, ...
. An idealized equation for this carbothermal reaction is shown for calcium phosphate (although phosphate rock contains substantial amounts of fluoroapatite):
:
Other polyhedrane analogues
Although white phosphorus forms thetetrahedron
In geometry, a tetrahedron (: tetrahedra or tetrahedrons), also known as a triangular pyramid, is a polyhedron composed of four triangular Face (geometry), faces, six straight Edge (geometry), edges, and four vertex (geometry), vertices. The tet ...
, the simplest possible Platonic hydrocarbon, no other polyhedral phosphorus clusters are known. White phosphorus converts to the thermodynamically-stabler red allotrope, but that allotrope is not isolated polyhedra.
Cubane
Cubane is a synthetic hydrocarbon compound with the Chemical formula, formula . It consists of eight carbon atoms arranged at the corners of a Cube (geometry), cube, with one hydrogen atom attached to each carbon atom. A solid crystalline substanc ...
, in particular, is unlikely to form, and the closest approach is the half-phosphorus compound , produced from phosphaalkynes. Other clusters are more thermodynamically favorable, and some have been partially formed as components of larger polyelemental compounds.
Red phosphorus
 Red phosphorus may be formed by heating
Red phosphorus may be formed by heating white phosphorus
White phosphorus, yellow phosphorus, or simply tetraphosphorus (P4) is an allotrope of phosphorus. It is a translucent waxy solid that quickly yellows in light (due to its photochemical conversion into red phosphorus), and impure white phospho ...
to in the absence of air or by exposing white phosphorus to sunlight
Sunlight is the portion of the electromagnetic radiation which is emitted by the Sun (i.e. solar radiation) and received by the Earth, in particular the visible spectrum, visible light perceptible to the human eye as well as invisible infrare ...
. Red phosphorus exists as an amorphous
In condensed matter physics and materials science, an amorphous solid (or non-crystalline solid) is a solid that lacks the long-range order that is a characteristic of a crystal. The terms "glass" and "glassy solid" are sometimes used synonymousl ...
network. Upon further heating, the amorphous red phosphorus crystallizes. It has two crystalline forms: violet phosphorus
Elemental phosphorus can exist in several allotropy, allotropes, the most common of which are white phosphorus, white and red phosphorus, red solids. Solid violet and black allotropes are also known. Gaseous phosphorus exists as diphosphorus and ...
and fibrous red phosphorus. Bulk red phosphorus does not ignite in air at temperatures below , whereas pieces of white phosphorus ignite at about .
Under standard conditions it is more stable than white phosphorus, but less stable than the thermodynamically stable black phosphorus. The standard enthalpy of formation of red phosphorus is −17.6 kJ/mol. Red phosphorus is kinetically most stable.
It was first presented by Anton von Schrötter before the Vienna Academy of Sciences on December 9, 1847, although others had doubtlessly had this substance in their hands before, such as Berzelius.
Applications
Red phosphorus can be used as a very effectiveflame retardant
Flame retardants are a diverse group of chemicals that are added to manufactured materials, such as plastics and textiles, and surface finishes and coatings. Flame retardants are activated by the presence of an combustion, ignition source and pr ...
, especially in thermoplastic
A thermoplastic, or thermosoftening plastic, is any plastic polymer material that becomes pliable or moldable at a certain elevated temperature and solidifies upon cooling.
Most thermoplastics have a high molecular weight. The polymer chains as ...
s (e.g. polyamide
A polyamide is a polymer with repeating units linked by amide bonds.
Polyamides occur both naturally and artificially. Examples of naturally occurring polyamides are proteins, such as wool and silk. Artificially made polyamides can be made throug ...
) and thermosets (e.g. epoxy resins
Epoxy is the family of basic components or Curing (chemistry), cured end products of epoxy Resin, resins. Epoxy resins, also known as polyepoxides, are a class of reactive prepolymers and polymers which contain epoxide groups. The epoxide fun ...
or polyurethane
Polyurethane (; often abbreviated PUR and PU) is a class of polymers composed of organic chemistry, organic units joined by carbamate (urethane) links. In contrast to other common polymers such as polyethylene and polystyrene, polyurethane term ...
s). The flame retarding effect is based on the formation of polyphosphoric acid
In chemistry, a phosphoric acid, in the general sense, is a phosphorus acid, phosphorus oxoacid in which each phosphorus (P) atom is in the oxidation state +5, and is chemical bond, bonded to four oxygen (O) atoms, one of them through a double b ...
. Together with the organic polymer material, these acids create a char that prevents the propagation of the flames. The safety risks associated with phosphine
Phosphine (IUPAC name: phosphane) is a colorless, flammable, highly toxic compound with the chemical formula , classed as a pnictogen hydride. Pure phosphine is odorless, but technical grade samples have a highly unpleasant odor like rotting ...
generation and friction sensitivity of red phosphorus can be effectively minimized by stabilization and micro-encapsulation
Microencapsulation is a process in which tiny particles or droplets are surrounded by a coating to give small capsules, with useful properties. In general, it is used to incorporate food ingredients, enzymes, cells or other materials on a micr ...
. For easier handling, red phosphorus is often used in form of dispersions or masterbatches in various carrier systems. However, for electronic/electrical systems, red phosphorus flame retardant has been effectively banned by major OEMs due to its tendency to induce premature failures. One persistent problem is that red phosphorus in epoxy molding compounds induces elevated leakage current in semiconductor devices. Another problem was acceleration of hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
reactions in PBT insulating material.
Red phosphorus can also be used in the illicit production of methamphetamine
Methamphetamine (contracted from ) is a potent central nervous system (CNS) stimulant that is mainly used as a recreational drug use, recreational or Performance-enhancing substance, performance-enhancing drug and less commonly as a secon ...
and Krokodil.
Red phosphorus can be used as an elemental photocatalyst
In chemistry, photocatalysis is the acceleration of a photoreaction in the presence of a photocatalyst, the excited state of which "repeatedly interacts with the reaction partners forming reaction intermediates and regenerates itself after each ...
for hydrogen formation from the water. They display a steady hydrogen evolution rates of 633 μmol/(h⋅g) by the formation of small-sized fibrous phosphorus.
Violet or Hittorf's phosphorus

red phosphorus
Red phosphorus is an Allotropes of phosphorus, allotrope of phosphorus. It is an amorphous polymeric red solid that is stable in air. It can be easily converted from white phosphorus under light or heating. It finds applications as matches and fir ...
. In 1865, Johann Wilhelm Hittorf heated red phosphorus in a sealed tube at 530 °C. The upper part of the tube was kept at 444 °C. Brilliant opaque monoclinic
In crystallography, the monoclinic crystal system is one of the seven crystal systems. A crystal system is described by three Vector (geometric), vectors. In the monoclinic system, the crystal is described by vectors of unequal lengths, as in t ...
, or rhombohedral
In geometry, a rhombohedron (also called a rhombic hexahedron or, inaccurately, a rhomboid) is a special case of a parallelepiped in which all six faces are congruent rhombus, rhombi. It can be used to define the rhombohedral lattice system, a Ho ...
, crystals sublimed as a result. Violet phosphorus can also be prepared by dissolving white phosphorus in molten lead
Lead () is a chemical element; it has Chemical symbol, symbol Pb (from Latin ) and atomic number 82. It is a Heavy metal (elements), heavy metal that is density, denser than most common materials. Lead is Mohs scale, soft and Ductility, malleabl ...
in a sealed tube at 500 °C for 18 hours. Upon slow cooling, Hittorf's allotrope crystallises out. The crystals can be revealed by dissolving the lead in dilute nitric acid
Nitric acid is an inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into nitrogen oxide, oxides of nitrogen. Most com ...
followed by boiling in concentrated hydrochloric acid
Hydrochloric acid, also known as muriatic acid or spirits of salt, is an aqueous solution of hydrogen chloride (HCl). It is a colorless solution with a distinctive pungency, pungent smell. It is classified as a acid strength, strong acid. It is ...
. In addition, a fibrous form exists with similar phosphorus cages. The lattice structure of violet phosphorus was presented by Thurn and Krebs in 1969. Imaginary frequencies, indicating the irrationalities or instabilities of the structure, were obtained for the reported violet structure from 1969. The single crystal of violet phosphorus was also produced. The lattice structure of violet phosphorus has been obtained by single-crystal ''x''-ray diffraction to be monoclinic with space group of ''P''2/''n'' (13) (''a'' = 9.210, ''b'' = 9.128, ''c'' = 21.893 Å, ''β'' = 97.776°CSD-1935087
. The optical band gap of the violet phosphorus was measured by diffuse reflectance spectroscopy to be around 1.7 eV. The thermal decomposition temperature was 52 °C higher than its black phosphorus counterpart. The violet phosphorene was easily obtained from both mechanical and solution exfoliation.
Reactions of violet phosphorus
Violet phosphorus does not ignite in air until heated to 300 °C and is insoluble in all solvents. It is not attacked byalkali
In chemistry, an alkali (; from the Arabic word , ) is a basic salt of an alkali metal or an alkaline earth metal. An alkali can also be defined as a base that dissolves in water. A solution of a soluble base has a pH greater than 7.0. The a ...
and only slowly reacts with halogens
The halogens () are a group (periodic table), group in the periodic table consisting of six chemically related chemical element, elements: fluorine (F), chlorine (Cl), bromine (Br), iodine (I), and the radioactive elements astatine (At) and ten ...
. It can be oxidised by nitric acid
Nitric acid is an inorganic compound with the formula . It is a highly corrosive mineral acid. The compound is colorless, but samples tend to acquire a yellow cast over time due to decomposition into nitrogen oxide, oxides of nitrogen. Most com ...
to phosphoric acid
Phosphoric acid (orthophosphoric acid, monophosphoric acid or phosphoric(V) acid) is a colorless, odorless phosphorus-containing solid, and inorganic compound with the chemical formula . It is commonly encountered as an 85% aqueous solution, ...
. Violet phosphorus ignites upon impact in air.
If it is heated in an atmosphere of inert gas, for example nitrogen
Nitrogen is a chemical element; it has Symbol (chemistry), symbol N and atomic number 7. Nitrogen is a Nonmetal (chemistry), nonmetal and the lightest member of pnictogen, group 15 of the periodic table, often called the Pnictogen, pnictogens. ...
or carbon dioxide
Carbon dioxide is a chemical compound with the chemical formula . It is made up of molecules that each have one carbon atom covalent bond, covalently double bonded to two oxygen atoms. It is found in a gas state at room temperature and at norma ...
, it sublimes and the vapour condenses as white phosphorus. If it is heated in a vacuum
A vacuum (: vacuums or vacua) is space devoid of matter. The word is derived from the Latin adjective (neuter ) meaning "vacant" or "void". An approximation to such vacuum is a region with a gaseous pressure much less than atmospheric pressur ...
and the vapour condensed rapidly, violet phosphorus is obtained. It would appear that violet phosphorus is a polymer
A polymer () is a chemical substance, substance or material that consists of very large molecules, or macromolecules, that are constituted by many repeat unit, repeating subunits derived from one or more species of monomers. Due to their br ...
of high relative molecular mass, which on heating breaks down into molecules. On cooling, these would normally dimerize
In chemistry, dimerization is the process of joining two identical or similar Molecular entity, molecular entities by Chemical bond, bonds. The resulting bonds can be either strong or weak. Many symmetrical chemical species are described as dim ...
to give molecules (i.e. white phosphorus) but, in a vacuum
A vacuum (: vacuums or vacua) is space devoid of matter. The word is derived from the Latin adjective (neuter ) meaning "vacant" or "void". An approximation to such vacuum is a region with a gaseous pressure much less than atmospheric pressur ...
, they link up again to form the polymeric violet allotrope.
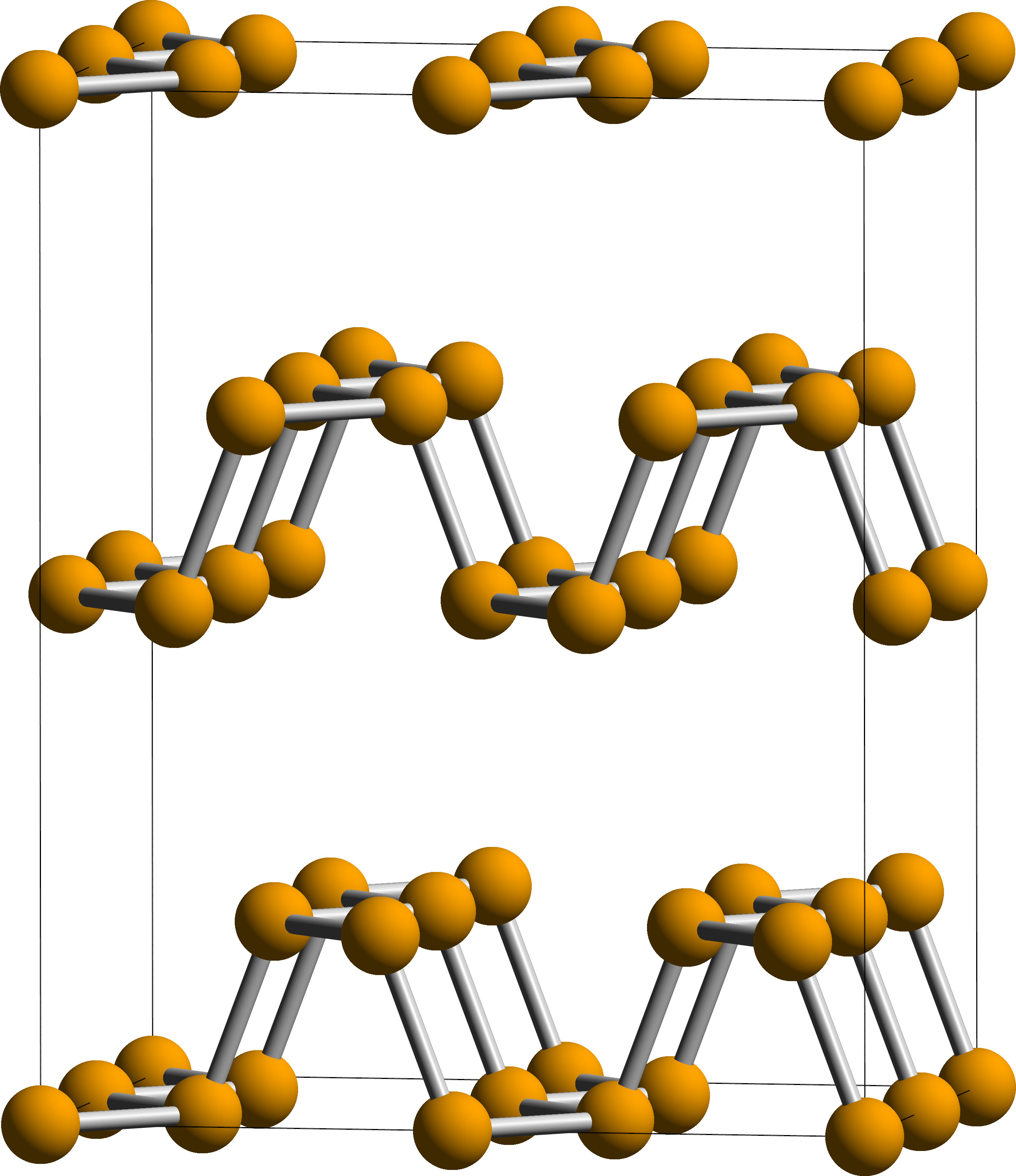
Black phosphorus


graphite
Graphite () is a Crystallinity, crystalline allotrope (form) of the element carbon. It consists of many stacked Layered materials, layers of graphene, typically in excess of hundreds of layers. Graphite occurs naturally and is the most stable ...
with both being black and flaky, a conductor of electricity, and having puckered sheets of linked atoms.
Black phosphorus has an orthorhombic
In crystallography, the orthorhombic crystal system is one of the 7 crystal systems. Orthorhombic Lattice (group), lattices result from stretching a cubic crystal system, cubic lattice along two of its orthogonal pairs by two different factors, res ...
pleated honeycomb structure and is the least reactive allotrope, a result of its lattice of interlinked six-membered rings where each atom is bonded to three other atoms. In this structure, each phosphorus atom has five outer shell electrons. Black and red phosphorus can also take a cubic
Cubic may refer to:
Science and mathematics
* Cube (algebra), "cubic" measurement
* Cube, a three-dimensional solid object bounded by six square faces, facets or sides, with three meeting at each vertex
** Cubic crystal system, a crystal system w ...
crystal lattice structure. The first high-pressure synthesis of black phosphorus crystals was made by the Nobel prize winner Percy Williams Bridgman in 1914. Metal salts catalyze the synthesis of black phosphorus.
Black phosphorus-based sensors exhibit several superior qualities over traditional materials used in piezoelectric or resistive sensors. Characterized by its unique puckered honeycomb lattice structure, black phosphorus provides exceptional carrier mobility. This property ensures its high sensitivity and mechanical resilience, making it an intriguing candidate for sensor technology.
Phosphorene
The similarities to graphite also include the possibility of scotch-tape delamination (exfoliation), resulting in phosphorene, agraphene
Graphene () is a carbon allotrope consisting of a Single-layer materials, single layer of atoms arranged in a hexagonal lattice, honeycomb planar nanostructure. The name "graphene" is derived from "graphite" and the suffix -ene, indicating ...
-like 2D material with excellent charge transport properties, thermal transport properties and optical properties. Distinguishing features of scientific interest include a thickness dependent band-gap, which is not found in graphene. This, combined with a high on/off ratio of ~105 makes phosphorene a promising candidate for field-effect transistors (FETs). The tunable bandgap also suggests promising applications in mid-infrared photodetectors and LEDs. Exfoliated black phosphorus sublimes at 400 °C in vacuum. It gradually oxidizes when exposed to water in the presence of oxygen, which is a concern when contemplating it as a material for the manufacture of transistors, for example. Exfoliated black phosphorus is an emerging anode material in the battery community, showing high stability and lithium
Lithium (from , , ) is a chemical element; it has chemical symbol, symbol Li and atomic number 3. It is a soft, silvery-white alkali metal. Under standard temperature and pressure, standard conditions, it is the least dense metal and the ...
storage.
Ring-shaped phosphorus
Ring-shaped phosphorus was theoretically predicted in 2007. The ring-shaped phosphorus was self-assembled inside evacuated multi-walled carbon nanotubes with inner diameters of 5–8 nm using a vapor encapsulation method. A ring with a diameter of 5.30 nm, consisting of 23 and 23 units with a total of 230 P atoms, was observed inside a multi-walled carbon nanotube with an inner diameter of 5.90 nm in atomic scale. The distance between neighboring rings is 6.4 Å. The ring shaped molecule is not stable in isolation.Blue phosphorus
Single-layer blue phosphorus was first produced in 2016 by the method of molecular beam epitaxy from black phosphorus as precursor.Diphosphorus

 The diphosphorus allotrope () can normally be obtained only under extreme conditions (for example, from at 1100 kelvin). In 2006, the diatomic molecule was generated in homogeneous solution under normal conditions with the use of
The diphosphorus allotrope () can normally be obtained only under extreme conditions (for example, from at 1100 kelvin). In 2006, the diatomic molecule was generated in homogeneous solution under normal conditions with the use of transition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. The lanthanide and actinid ...
complexes (for example, tungsten
Tungsten (also called wolfram) is a chemical element; it has symbol W and atomic number 74. It is a metal found naturally on Earth almost exclusively in compounds with other elements. It was identified as a distinct element in 1781 and first ...
and niobium
Niobium is a chemical element; it has chemical symbol, symbol Nb (formerly columbium, Cb) and atomic number 41. It is a light grey, crystalline, and Ductility, ductile transition metal. Pure niobium has a Mohs scale of mineral hardness, Mohs h ...
).
Diphosphorus is the gaseous form of phosphorus
Phosphorus is a chemical element; it has Chemical symbol, symbol P and atomic number 15. All elemental forms of phosphorus are highly Reactivity (chemistry), reactive and are therefore never found in nature. They can nevertheless be prepared ar ...
, and the thermodynamically stable form between 1200 °C and 2000 °C. The dissociation of tetraphosphorus () begins at lower temperature: the percentage of at 800 °C is ≈ 1%. At temperatures above about 2000 °C, the diphosphorus molecule begins to dissociate into atomic phosphorus.
Phosphorus nanorods
nanorod polymers were isolated from CuI-P complexes using low temperature treatment. Red/brown phosphorus was shown to be stable in air for several weeks and have properties distinct from those of red phosphorus.Electron microscopy
An electron microscope is a microscope that uses a beam of electrons as a source of illumination. It uses electron optics that are analogous to the glass lenses of an optical light microscope to control the electron beam, for instance focusing i ...
showed that red/brown phosphorus forms long, parallel nanorods with a diameter between 3.4 Å and 4.7 Å.
Properties
See also
* Phossy jawReferences
{{reflist, 30emExternal links
;White phosphorusWhite Phophorus
at ''
The Periodic Table of Videos
''Periodic Videos'' (also known as ''The Periodic Table of Videos'') is a video project and YouTube channel on chemistry. It consists of a series of videos about chemical elements and the periodic table, with additional videos on other topics i ...
'' (University of Nottingham)
More about White Phosphorus (and phosphorus pentoxide)
at ''
The Periodic Table of Videos
''Periodic Videos'' (also known as ''The Periodic Table of Videos'') is a video project and YouTube channel on chemistry. It consists of a series of videos about chemical elements and the periodic table, with additional videos on other topics i ...
'' (University of Nottingham)The Chemistry of Phosphorus
at Chemistry LibreTexts.
Phosphorus
Phosphorus is a chemical element; it has Chemical symbol, symbol P and atomic number 15. All elemental forms of phosphorus are highly Reactivity (chemistry), reactive and are therefore never found in nature. They can nevertheless be prepared ar ...
Phosphorus