Victor Grignard on:
[Wikipedia]
[Google]
[Amazon]
Francois Auguste Victor Grignard (6 May 1871 – 13 December 1935) was a French
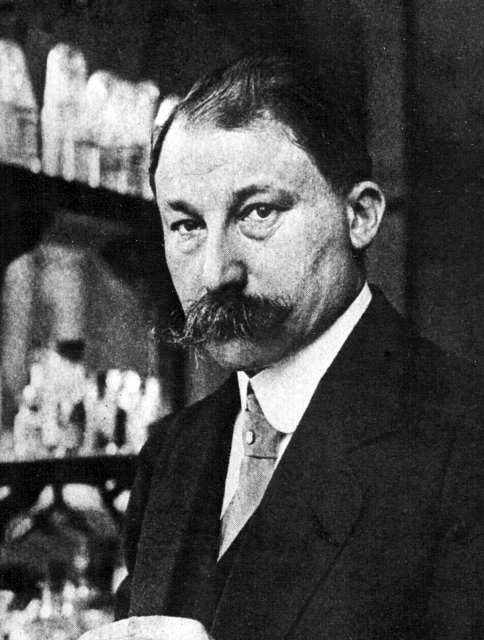 Grignard was the son of a sailmaker. He was a hard-working student and was described as having a humble and friendly attitude. He also had a talent for mathematics. After attempting to major in mathematics, Grignard failed his entrance exams before being drafted into the army in 1892. After one year of service, he returned to pursue his studies of mathematics at the University of Lyon and finally obtained his degree Licencié ès Sciences Mathématiques in 1894. In December of the same year, he transferred to chemistry and began working with Professors Philippe Barbier (1848–1922) and Louis Bouveault (1864–1909). After working with
Grignard was the son of a sailmaker. He was a hard-working student and was described as having a humble and friendly attitude. He also had a talent for mathematics. After attempting to major in mathematics, Grignard failed his entrance exams before being drafted into the army in 1892. After one year of service, he returned to pursue his studies of mathematics at the University of Lyon and finally obtained his degree Licencié ès Sciences Mathématiques in 1894. In December of the same year, he transferred to chemistry and began working with Professors Philippe Barbier (1848–1922) and Louis Bouveault (1864–1909). After working with
Comptes Rendus
{{DEFAULTSORT:Grignard, Victor 1871 births 1935 deaths 19th-century French chemists French organic chemists Nobel laureates in Chemistry Members of the French Academy of Sciences French Nobel laureates Chemical warfare People from Manche Academic staff of Nancy-Université Commanders of the Legion of Honour 20th-century French chemists
chemist
A chemist (from Greek ''chēm(ía)'' alchemy; replacing ''chymist'' from Medieval Latin ''alchemist'') is a graduated scientist trained in the study of chemistry, or an officially enrolled student in the field. Chemists study the composition of ...
who won the Nobel Prize
The Nobel Prizes ( ; ; ) are awards administered by the Nobel Foundation and granted in accordance with the principle of "for the greatest benefit to humankind". The prizes were first awarded in 1901, marking the fifth anniversary of Alfred N ...
for his discovery of the eponymously named Grignard reagent and Grignard reaction, both of which are important in the formation of carbon–carbon bonds. He also wrote some of his experiments in his laboratory notebooks.
Biography
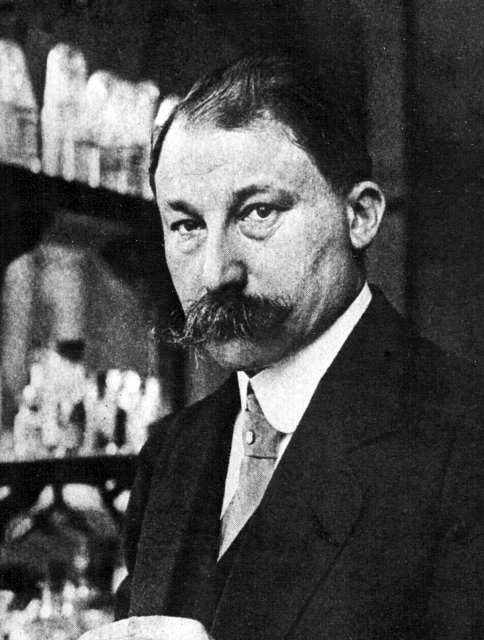 Grignard was the son of a sailmaker. He was a hard-working student and was described as having a humble and friendly attitude. He also had a talent for mathematics. After attempting to major in mathematics, Grignard failed his entrance exams before being drafted into the army in 1892. After one year of service, he returned to pursue his studies of mathematics at the University of Lyon and finally obtained his degree Licencié ès Sciences Mathématiques in 1894. In December of the same year, he transferred to chemistry and began working with Professors Philippe Barbier (1848–1922) and Louis Bouveault (1864–1909). After working with
Grignard was the son of a sailmaker. He was a hard-working student and was described as having a humble and friendly attitude. He also had a talent for mathematics. After attempting to major in mathematics, Grignard failed his entrance exams before being drafted into the army in 1892. After one year of service, he returned to pursue his studies of mathematics at the University of Lyon and finally obtained his degree Licencié ès Sciences Mathématiques in 1894. In December of the same year, he transferred to chemistry and began working with Professors Philippe Barbier (1848–1922) and Louis Bouveault (1864–1909). After working with stereochemistry
Stereochemistry, a subdiscipline of chemistry, studies the spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereoisomers, which are defined ...
and énines, Grignard was not impressed with the subject matter and asked Barbier about a new direction for his doctoral research. Barbier advised that Grignard study how a failed Saytzeff reaction using zinc
Zinc is a chemical element; it has symbol Zn and atomic number 30. It is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic tabl ...
, was successful, in low yields, after substitution of magnesium
Magnesium is a chemical element; it has Symbol (chemistry), symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 ...
. They sought to synthesize alcohols from alkyl halides, aldehydes, ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
s, and alkenes. Grignard hypothesized that the aldehyde or ketone prevented the magnesium from reacting with the alkyl halide, accounting for the low yields. He tested his hypothesis by first adding an alkyl halide and magnesium filings to a solution of anhydrous ether and then adding the aldehyde or ketone. This resulted in a drastic increase in the yield of the reaction.
A couple of years later, Grignard was able to isolate the intermediate. He had heated a mixture of magnesium turnings and isobutyl iodide and added dry ethyl ether to the mixture, observing the reaction. The product is known as a Grignard reagent. Named after him, this organo-magnesium compound (R-MgX) (R = alkyl ; X = Halogen) readily reacts with ketones, aldehydes, and alkenes to produce their respective alcohols in impressive yields. Grignard had discovered the synthetic reaction that now bears his name (the Grignard reaction) in 1900. In 1901, he published his doctoral thesis titled "Thèses sur les combinaisons organomagnesiennes mixtes et leur application à des synthèses d‘acides, d‘alcools et d‘hydrocarbures". He became a lecturer in organic chemistry at the University of Nancy in 1909, and was promoted to full professor in 1910. In 1912 he and Paul Sabatier (1854–1941) were awarded the Nobel Prize in Chemistry. During World War I
World War I or the First World War (28 July 1914 – 11 November 1918), also known as the Great War, was a World war, global conflict between two coalitions: the Allies of World War I, Allies (or Entente) and the Central Powers. Fighting to ...
he studied chemical warfare agents with Georges Urbain at Sorbonne University, particularly the manufacture of phosgene and the detection of mustard gas. In 1918, Grignard discovered that sodium iodide could be used as a battlefield test for mustard gas. Sodium iodide converts mustard gas to diiododiethyl sulfide, which crystallizes more easily than mustard gas. This test could detect as little as 0.01 gram of mustard gas in one cubic meter of air and was successfully used on the battlefield. His counterpart on the German side was another Nobel Prize–winning chemist, Fritz Haber.
Grignard died on 13 December 1935 in Lyon
Lyon (Franco-Provençal: ''Liyon'') is a city in France. It is located at the confluence of the rivers Rhône and Saône, to the northwest of the French Alps, southeast of Paris, north of Marseille, southwest of Geneva, Switzerland, north ...
, at the age of 64. By that time, around 6,000 papers reporting applications of the Grignard reaction had been published.
Grignard reaction
Grignard is most noted for devising a new method for generating carbon-carbon bonds using magnesium to couple ketones and alkyl halides. This reaction is valuable in organic synthesis. It occurs in two steps: #Formation of the " Grignard reagent", which is an organomagnesium compound made by the reaction of an organohalide, R-X (R =alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl group is derived from a cy ...
or aryl; and X is a halide, usually bromide or iodide) with magnesium
Magnesium is a chemical element; it has Symbol (chemistry), symbol Mg and atomic number 12. It is a shiny gray metal having a low density, low melting point and high chemical reactivity. Like the other alkaline earth metals (group 2 ...
metal. The Grignard reagent is usually described with the general chemical formula R-Mg-X, although its structure is more complex.
#Addition of the carbonyl, in which a ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
or an aldehyde is added to the solution containing the Grignard reagent. The carbon atom that is bonded to Mg transfers to the carbonyl carbon atom, and the oxygen of the carbonyl carbon becomes attached to the magnesium to give an alkoxide. The process is an example of a nucleophilic addition to a carbonyl. After the addition, the reaction mixture is treated with aqueous acid to give an alcohol, and the magnesium salts are subsequently discarded.
Military service
Grignard was drafted into the French military as part of obligatory military service in 1892. Within the two years of his first session of service he rose to the rank of corporal. He was demobilized in 1894 and returned to Lyon to pursue his education. He was awarded a medal of theLegion of Honour
The National Order of the Legion of Honour ( ), formerly the Imperial Order of the Legion of Honour (), is the highest and most prestigious French national order of merit, both military and Civil society, civil. Currently consisting of five cl ...
and made a Chevalier in 1912 after winning the Nobel Prize. When World War I broke out, Grignard was drafted back into the military, keeping his rank of corporal. He was placed on sentry duty, and served there for several months until he was brought to the attention of the General Staff. Grignard had been wearing his Medal of the Legion of Honour, despite being ordered to take it off by a superior. After looking more into Grignard, the General Staff decided that he would be better suited for research than sentry duty, so they assigned him to the explosives division. Grignard's research shifted to antidotes to chemical weapons when production of TNT was no longer sustainable, and eventually Grignard was assigned to research new chemical weapons for the French army.
Honors
* 1912: Nobel Prize in Chemistry for his discovery of the Grignard reagent (shared the award with fellow Frenchman Paul Sabatier). * 1912: Lavoisier Medal, Société Chimique de France * 1933: Légion d'Honneur, CommanderNotes
References
* * * *External links
* including the Nobel Lecture, 11 December 1912 ''The Use of Organomagnesium Compunds in Preparative Organic Chemistry''Comptes Rendus
{{DEFAULTSORT:Grignard, Victor 1871 births 1935 deaths 19th-century French chemists French organic chemists Nobel laureates in Chemistry Members of the French Academy of Sciences French Nobel laureates Chemical warfare People from Manche Academic staff of Nancy-Université Commanders of the Legion of Honour 20th-century French chemists