Turbo-Hauser Bases on:
[Wikipedia]
[Google]
[Amazon]
''Turbo''-Hauser bases are amido magnesium halides that contain stoichiometric amounts of LiCl. These mixed Mg/Li
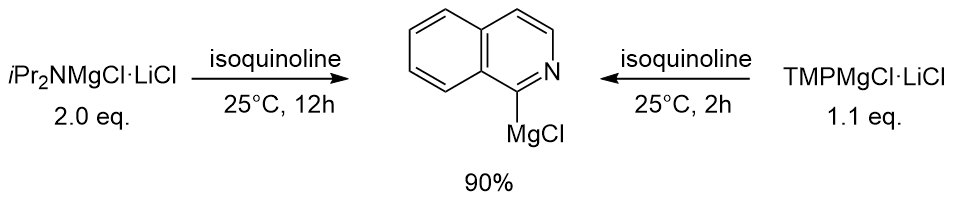
 Another difference is illustrated by the differing rates of deprotonation of isoquinoline in THF solution. Whereas TMPMgCl·LiCl required only 2h and 1.1 equivalents, ''i''Pr2NMgCl·LiCl needed 12h and 2 equivalents for comparable metalation.
Another difference is illustrated by the differing rates of deprotonation of isoquinoline in THF solution. Whereas TMPMgCl·LiCl required only 2h and 1.1 equivalents, ''i''Pr2NMgCl·LiCl needed 12h and 2 equivalents for comparable metalation.
 The differing reactivity of the TMP vs iPr-based reagents is related to the fact that the TMP is always a terminal ligand whereas iPr2N is sometimes bridging (μ-). Generally, in
The differing reactivity of the TMP vs iPr-based reagents is related to the fact that the TMP is always a terminal ligand whereas iPr2N is sometimes bridging (μ-). Generally, in
amide
In organic chemistry, an amide, also known as an organic amide or a carboxamide, is a chemical compound, compound with the general formula , where R, R', and R″ represent any group, typically organyl functional group, groups or hydrogen at ...
s of the type R2NMgCl⋅LiCl are used in organic chemistry as non-nucleophilic bases for metalation reactions of aromatic
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated system, conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugati ...
and heteroaromatic
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugation alone. The ea ...
substrates. Compared to their LiCl free ancestors ''Turbo''-Hauser bases show an enhanced kinetic basicity
In chemistry, there are three definitions in common use of the word "base": ''Arrhenius bases'', ''Brønsted bases'', and ''Lewis bases''. All definitions agree that bases are substances that react with acids, as originally proposed by Guilla ...
, excellent regioselectivity, high functional group tolerance and a better solubility.
Preparation
Typically ''Turbo''-Hauser bases are prepared by treating anamine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
with a Grignard reagent
Grignard reagents or Grignard compounds are chemical compounds with the general formula , where X is a halogen and R is an organic group, normally an alkyl or aryl. Two typical examples are methylmagnesium chloride and phenylmagnesium bromi ...
and lithium chloride
Lithium chloride is a chemical compound with the formula Li Cl. The salt is a typical ionic compound (with certain covalent characteristics), although the small size of the Li+ ion gives rise to properties not seen for other alkali metal chlorid ...
. In some cases they are prepared by treating a lithium amide
Lithium amide or lithium azanide is an inorganic compound with the chemical formula . It is a white solid with a tetragonal crystal structure. Lithium amide can be made by treating lithium metal with liquid ammonia:
:
Lithium amide decomposes into ...
with MgCl2:
:
:
Common ''Turbo''-Hauser bases: R'2NH = ''i''Pr2NMgCl·LiCl (''i''Pr-''Turbo''-Hauser base), TMPMgCl·LiCl, TMP (''Turbo''-Hauser base or ''Knochel''-Hauser Base)
Structure
In solution, ''Turbo''-Hauser bases participate in temperature- and concentration-dependent equilibria. Diffusion-Ordered Spectroscopy (DOSY) show that at room temperature and high concentrations (0.6 M) dimeric 'i''Pr2NMgCl·LiClsub>2 remains intact solution.Solid State Structure
The ''i''Pr-''Turbo''-Hauser base crystallizes as a dimeric amido bridgedcontact ion pair
In chemistry, the intimate ion pair concept, introduced by Saul Winstein, describes the interactions between a cation, anion and surrounding solvent molecules. In ordinary aqueous solutions of inorganic salts, an ion is completely solvated and sh ...
(CIP). Due to the high steric demand of the TMP TMP may refer to:
Arts and media
* Tickle Me Pink, a rock band from Colorado, US (2005–2011)
* Tiny Moving Parts, an emo band from Minnesota, US
* Tom Malone Prize, an Australian glass art prize
* Tsukuyomi -Moon Phase-, a 2000–2008 anime ...
ligand the dimerization process is sterically hindered. This is why the TMP-''Turbo''-Hauser base crystallizes as a monomeric CIP.
In both structures LiCl coordinates to the magnesium amides.
The solid state structure of TMPMgCl·LiCl is retained almost completely in THF solution independently of temperature and concentration. Due to the high steric demand of the TMP ligand, the THF ligand dissociates from the magnesium cation. This dissociation gives a magnesium amido complex with enhanced reactivity for deprotonation of C-H bonds.
:
Reactions
''Turbo''-Hauser bases are used asmetalation
Metalation (Alt. spelling: Metallation) is a chemical reaction that forms a bond to a metal. This reaction usually refers to the replacement of a halogen atom in an organic molecule with a metal atom, resulting in an organometallic compound. In the ...
/deprotonation
Deprotonation (or dehydronation) is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction.Henry Jakubowski, Biochemistry Online Chapter 2A3, https://employees.csbsju.ed ...
reagents. In this way, they resemble some organolithium reagent
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom ...
s. The lithiated compounds, however, are only stable at low temperatures (e.g. -78 °C) and suffer competing addition reactions (like e.g. Chichibabin reactions). In contrast, the magnesium compounds are less reactive. The magnesium amide complex is stabilized by LiCl. ''Turbo''-Hauser bases display a high functional group tolerance and greater chemoselectivity at high and low temperatures. The resulting reagent is then quenched with an electrophile.
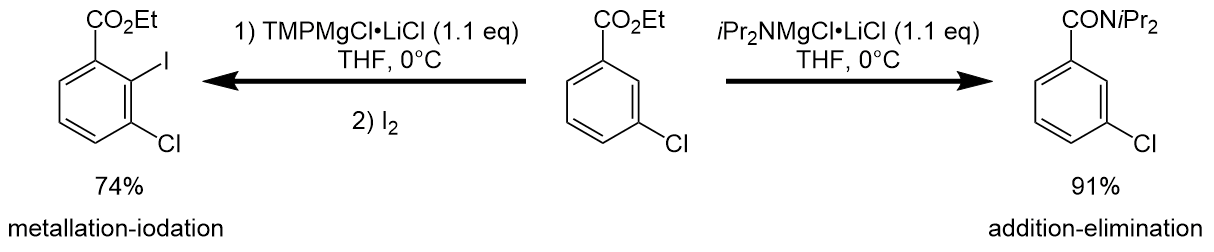
''i''Pr2NMgCl·LiCl and TMPMgCl·LiCl react differently. The TMP-''Turbo''-Hauser base easily metalates ethyl-3-chlorobenzoate in the C2 position, while the same reaction carried out with the ''i''Pr-''Turbo''-Hauser base resulted in no metalation at all. Instead, an addition-elimination reaction occurs.
 Another difference is illustrated by the differing rates of deprotonation of isoquinoline in THF solution. Whereas TMPMgCl·LiCl required only 2h and 1.1 equivalents, ''i''Pr2NMgCl·LiCl needed 12h and 2 equivalents for comparable metalation.
Another difference is illustrated by the differing rates of deprotonation of isoquinoline in THF solution. Whereas TMPMgCl·LiCl required only 2h and 1.1 equivalents, ''i''Pr2NMgCl·LiCl needed 12h and 2 equivalents for comparable metalation.
 The differing reactivity of the TMP vs iPr-based reagents is related to the fact that the TMP is always a terminal ligand whereas iPr2N is sometimes bridging (μ-). Generally, in
The differing reactivity of the TMP vs iPr-based reagents is related to the fact that the TMP is always a terminal ligand whereas iPr2N is sometimes bridging (μ-). Generally, in organolithium
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom ...
chemistry monomeric species display the most active kinetic species. This could explain why reactions of the monomeric TMP-''Turbo''-Hauser base are much faster than that of dimeric ''i''Pr-''Turbo''-Hauser base. The regioselective ortho deprotonation reactions of TMPMgCl·LiCl could stem from a sufficient complex-induced proximity effect (CIPE) between the bimetallic aggregate and the functionalized (hetero)aromatic substrate.
Related reagents
* Turbo-Grignard reagents used for halide/Mg exchange reactions. "Turbo-Grignards", as they are often called, are aggregates with the formula -PrMgCl·LiClsub>2 *Organozinc-LiCl complexesReferences
{{Reflist Magnesium compounds *