Trityl Cation on:
[Wikipedia]
[Google]
[Amazon]
 In
In
Image:Methyl Violet 10B.png,
N. C. Deno, J. J. Jaruzelski, and Alan Schriesheim (1955) "Carbonium ions. I. An acidity function (''C''0) derived from arylcarbonium ion equilibria." ''Journal of the American Chemical Society'', volume 77, issue 11, pages 3044–3051.
Michael E. Jung, Roman Lagoutte, and Ullrich Jahn (2011): "Triphenylcarbenium Tetrafluoroborate". In ''Encyclopedia of Reagents for Organic Synthesis''.
E. Molins, M. Mas, W. Maniukiewicz, M. Ballester and J. Castañer (1996): "Perchlorotriphenylcarbenium Hexachloroantimonate(V)". ''Acta Crystallographica Section C (Structural Chemistry)'', volume C52, pages 2412-2414. {{doi, 10.1107/S0108270196007287
U. S. National Institutes of Health (2019)
PubChem ID 2723954 - Triphenylcarbenium hexafluorophosphate
. Entry in NCBI's PubChem database, accessed on 2019-07-25.
Carbocations

 In
In chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules a ...
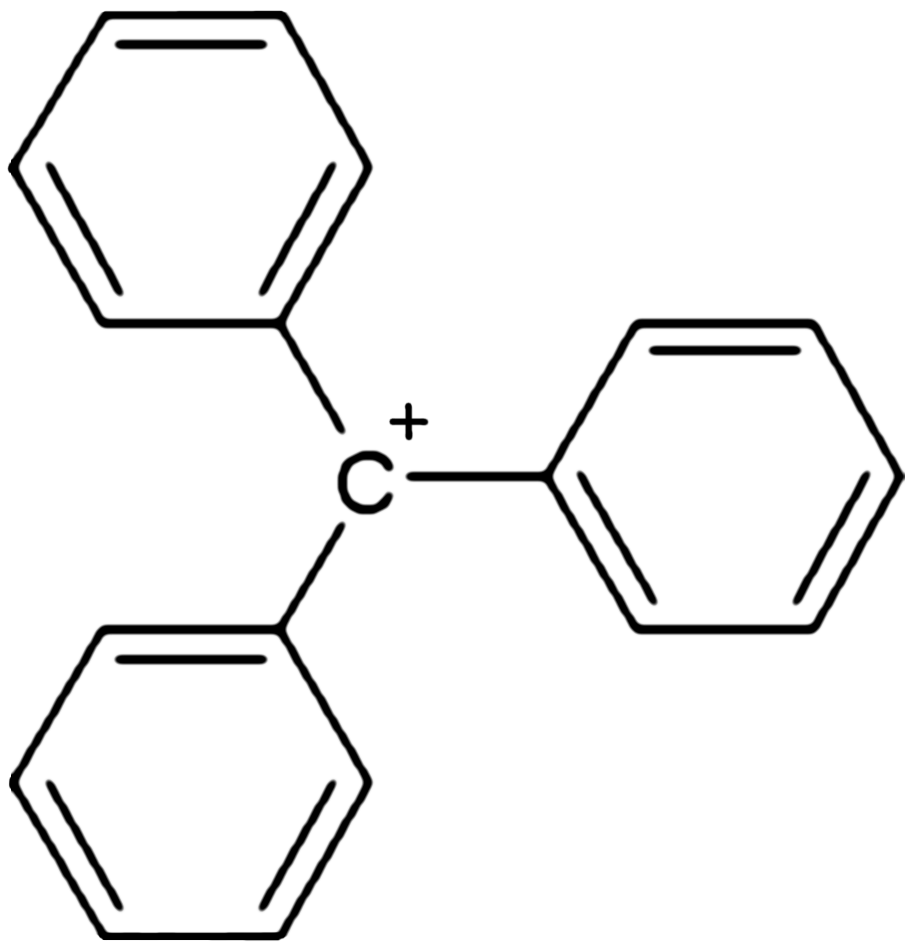
, triphenylcarbenium, triphenylmethyl cation, tritylium , or trityl cation is an ion
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
with formula or , consisting of a carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
atom with a positive charge
Electric charge (symbol ''q'', sometimes ''Q'') is a physical property of matter that causes it to experience a force when placed in an electromagnetic field. Electric charge can be ''positive'' or ''negative''. Like charges repel each other and ...
connected to three phenyl
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula , and is often represented by the symbol Ph (archaically φ) or Ø. The phenyl group is closely related to benzene and can be viewed as a benzene ...
groups. It is a charged version of the triphenylmethyl radical
The triphenylmethyl radical (often shortened to trityl radical after 1927 suggestion by Burckhardt Helferich, Helferich et al.) is an organic compound with the formula (C6H5)3C. It is a persistent radical. It was the first radical (chemistry), rad ...
. The name is often abbreviated to triphenylmethyl or trityl in salts, although these names also denote the chemical group in compounds like triphenylmethyl chloride
Triphenylmethyl chloride or trityl chloride (TrCl) is a white solid with the chemical formula C19H15Cl. It is an alkyl halide, sometimes used to introduce the trityl protecting group.
Preparation
Triphenylmethyl chloride is commercially available. ...
that do not contain the cation.
Triphenylcarbenium is a relatively stable carbenium
The carbenium ion is a kind of positive ion with the structure RR′R″C+, that is, a chemical species with carbon atom having three covalent bonds, and it bears a +1 formal charge. Carbenium ions are a major subset of carbocations, which is a ...
ion, because the positive charge is partially distributed among 10 of the carbon atoms (the 3 carbon atoms in the ''ortho'' and ''para'' positions of each of the three phenyl groups, plus the central carbon atom).
Derivatives
The cation exists in important chemicalreagent
In chemistry, a reagent ( ) or analytical reagent is a substance or compound added to a system to cause a chemical reaction, or test if one occurs. The terms ''reactant'' and ''reagent'' are often used interchangeably, but reactant specifies a ...
s and catalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
s such as triphenylmethyl hexafluorophosphate
Triphenylmethyl hexafluorophosphate (also triphenylcarbenium hexafluorophosphate, trityl hexafluorophosphate, or tritylium hexafluorophosphate) is an organic salt with the formula , consisting of the triphenylcarbenium cation and the hexafluorop ...
. Related salts are known with diverse anions including tetrafluoroborate
Tetrafluoroborate is the anion . This tetrahedral species is isoelectronic with tetrafluoroberyllate (), tetrafluoromethane (CF4), and tetrafluoroammonium () and is valence isoelectronic with many stable and important species including the perc ...
(), hexachloroantimonate
Antimony pentachloride is a chemical compound with the formula SbCl5. It is a colourless oil, but typical samples are yellowish due to dissolved chlorine. Owing to its tendency to hydrolyse to hydrochloric acid, SbCl5 is a highly corrosive subst ...
(), and perchlorate
A perchlorate is a chemical compound containing the perchlorate ion, , the conjugate base of perchloric acid (ionic perchlorate). As counterions, there can be metal cations, quaternary ammonium cations or other ions, for example, nitronium cat ...
(). This and other similar cations can be obtained as intensely colored solutions by dissolving aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used ...
-substituted methanol
Methanol (also called methyl alcohol and wood spirit, amongst other names) is an organic chemical compound and the simplest aliphatic Alcohol (chemistry), alcohol, with the chemical formula (a methyl group linked to a hydroxyl group, often ab ...
s in concentrated sulfuric acid
Sulfuric acid (American spelling and the preferred IUPAC name) or sulphuric acid (English in the Commonwealth of Nations, Commonwealth spelling), known in antiquity as oil of vitriol, is a mineral acid composed of the elements sulfur, oxygen, ...
. Derivatives of this cation include, for example, perchlorotriphenylcarbenium .
Triarylmethane dyes
Triarylmethane dye
Triarylmethane dyes are synthetic organic compounds containing triphenylmethane backbones. As dyes, these compounds are intensely colored. They are produced industrially as dyes.
Families
Triarylmethane dyes can be grouped into families accordin ...
s are derivatives that are stabilized versions of the trityl cation. They are water-soluble and are often obtained as the chloride salts. These dyes have strong electron donor groups, often amines, at the ''p''-positions of two or three of the aryl groups.Thomas Gessner and Udo Mayer "Triarylmethane and Diarylmethane Dyes" in Ullmann's Encyclopedia of Industrial Chemistry
''Ullmann's Encyclopedia of Industrial Chemistry'' is a major reference work related to Chemical industry, industrial chemistry by chemist Fritz Ullmann, first published in 1914, and exclusively in German as "Enzyklopädie der Technischen Chemie ...
2002, Wiley-VCH
Wiley-VCH is a German publisher owned by John Wiley & Sons. It was founded in 1921 as Verlag Chemie (meaning "Chemistry Press": VCH stands for ''Verlag Chemie'') by two German learned societies
A learned society ( ; also scholarly, intellect ...
, Weinheim.
Crystal violet
Crystal violet or gentian violet, also known as methyl violet 10B or hexamethyl pararosaniline chloride, is a triphenylmethane, triarylmethane dye used as a histological stain and in Gram staining, Gram's method of classifying bacteria. Crystal ...
.
Image:NewFuchsineStructure.png, New fuchsine
New fuchsine is an organic compound with the formula H2N(CH3)C6H3)3Cl. It is a green-colored solid that is used as a dye of the triarylmethane class. It is one of the four components of basic fuchsine, and one of the two that are available as ...
dye.
File:Pararosaniline.png, Pararosaniline
Pararosaniline, pararosaniline free base, Basic Red 9, or C.I. 42500 is an organic compound with the formula . It is the free base form of pararosaniline hydrochloride, , a magenta solid with a variety of uses as a dye. It is one of the four comp ...
See also
*Triphenylmethane
Triphenylmethane or triphenyl methane (sometimes also known as Tritan), is the hydrocarbon with the formula (C6H5)3CH. This colorless solid is soluble in nonpolar organic solvents and not in water. Triphenylmethane is the basic skeleton of many syn ...
*Triphenylmethanol
Triphenylmethanol (also known as triphenylcarbinol and TrOH) is an organic compound. It is a white crystalline solid that is insoluble in water and petroleum ether, but well soluble in ethanol, diethyl ether, and benzene. In strongly acidic soluti ...
References
PubChem ID 2723954 - Triphenylcarbenium hexafluorophosphate
. Entry in NCBI's PubChem database, accessed on 2019-07-25.