Trisoxazolines on:
[Wikipedia]
[Google]
[Amazon]
 Trisoxazolines (Often abbreviated TRISOX or TOX) are a class of tridentate,
Trisoxazolines (Often abbreviated TRISOX or TOX) are a class of tridentate,
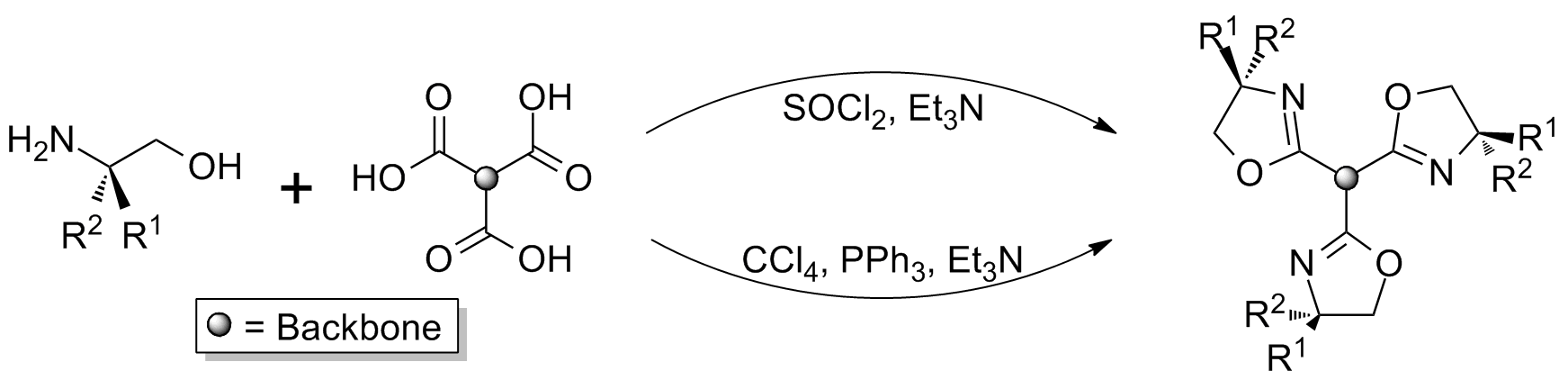
 In addition to the inclusion of heterochirality, modular synthesis also allows for the synthesis of 'lopsided' structures which have application as
In addition to the inclusion of heterochirality, modular synthesis also allows for the synthesis of 'lopsided' structures which have application as 
 Trisoxazolines (Often abbreviated TRISOX or TOX) are a class of tridentate,
Trisoxazolines (Often abbreviated TRISOX or TOX) are a class of tridentate, chiral ligand
Enantioselective synthesis, also called asymmetric synthesis, is a form of chemical synthesis. It is defined by IUPAC as "a chemical reaction (or reaction sequence) in which one or more new elements of chirality are formed in a substrate molecul ...
s composed of three oxazoline
Oxazoline is a five-membered heterocyclic organic compound with the formula . It is the parent of a family of compounds called oxazolines (emphasis on plural), which contain non-hydrogenic substituents on carbon and/or nitrogen. Oxazolines are the ...
rings. Despite being neutral they are able to form stable complexes with high oxidation state metals, such as rare earths
The rare-earth elements (REE), also called the rare-earth metals or rare earths, and sometimes the lanthanides or lanthanoids (although scandium and yttrium, which do not belong to this series, are usually included as rare earths), are a set of ...
, due to the chelate effect
Chelation () is a type of bonding of ions and their molecules to metal ions. It involves the formation or presence of two or more separate coordinate bonds between a polydentate (multiple bonded) ligand and a single central metal atom. These l ...
. The ligands have been investigated for molecular recognition
Supramolecular chemistry refers to the branch of chemistry concerning Chemical species, chemical systems composed of a integer, discrete number of molecules. The strength of the forces responsible for spatial organization of the system range from w ...
and their complexes are used in asymmetric catalysts and polymerisation
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many form ...
.
Synthesis
Trisoxazolines can either be synthesised directly, from suitable tripodal starting materials, or built up in a modular manner. These approaches can be used to give ligands of differing symmetries, with the direct synthesis route giving homochiral ligands with C3 rotational symmetry and the modular approach typically being used to give asymmetric compounds (C1 symmetry), which are either heterochiral or possess a mix of bothchiral
Chirality () is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek language, Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is dist ...
and achiral groups. These differences in symmetry can significantly effect the coordination chemistry of the ligands and the catalytic activity of their complexes, with C3 symmetric ligands often being better for asymmetric catalysis
Enantioselective synthesis, also called asymmetric synthesis, is a form of chemical synthesis. It is defined by IUPAC as "a chemical reaction (or reaction sequence) in which one or more new elements of Chirality (chemistry), chirality are formed ...
.
Direct methods
Suitable tripodal compounds, such astrimesic acid
Trimesic acid, also known as benzene-1,3,5-tricarboxylic acid, is an organic compound with the formula C6H3(CO2H)3. It is one of three isomers of benzenetricarboxylic acid. A colorless solid, trimesic acid has some commercial value as a precursor ...
and nitrilotriacetic acid
Nitrilotriacetic acid (NTA) is the aminopolycarboxylic acid with the formula N(CH2CO2H)3. It is a colourless solid. Its conjugate base nitrilotriacetate is used as a chelating agent for Ca2+, Co2+, Cu2+, and Fe3+.
Production and use
Nitrilotria ...
, can be converted directly to trisoxazolines. The simplicity of this approach is beneficial, however it only allows a limited variety of structures to be produced, due to the limited range of available starting materials.
:
Modular methods
Modular synthesis allows for a more diverse range of structures, however the multi-step reactions can result in lower overall yields. In general synthesis involves the generation of separate mono‑oxazoline (typically halogenated) and bis-oxazoline units, which are then coupled using a strong base such as tBuLi or KN(SiMe3)2. : In addition to the inclusion of heterochirality, modular synthesis also allows for the synthesis of 'lopsided' structures which have application as
In addition to the inclusion of heterochirality, modular synthesis also allows for the synthesis of 'lopsided' structures which have application as scorpionate ligand
In coordination chemistry, a scorpionate ligand is a tridentate (three-donor-site) ligand that binds to a central atom in a ''fac'' manner. The most popular class of scorpionates are the hydrotris(pyrazolyl)borates or Tp ligands. These were al ...
s.
:
In catalysis
Friedel–Crafts reaction
Trisoxazolines have been used for the copper catalysed Friedel–Crafts alkylation ofindole
Indole is an organic compound with the formula . Indole is classified as an aromatic heterocycle. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered pyrrole ring. Indoles are derivatives of indole ...
s, largely with alkylidene malonates, with good yields and ee's reported. A number of interesting solvent effects
In chemistry, solvent effects are the influence of a solvent on chemical reactivity or molecular associations. Solvents can have an effect on solubility, stability and reaction rates and choosing the appropriate solvent allows for thermodynami ...
have also been observed, including a relationship between enantioselectivity
In chemistry, stereoselectivity is the property of a chemical reaction in which a single reactant forms an unequal mixture of stereoisomers during a non- stereospecific creation of a new stereocenter or during a non-stereospecific transformation o ...
and the steric bulk
Steric effects arise from the spatial arrangement of atoms. When atoms come close together there is generally a rise in the energy of the molecule. Steric effects are nonbonding interactions that influence the shape ( conformation) and reactivi ...
of the solvent when using of alcohols and a reversal of enantioselectivity when changing the reaction solvent from coordinating solvents to weakly coordinating solvents.
:
Polymerisation
Rare-earth complexes incorporating TRISOX ligands have been found to be highly effective catalysts for thepolymerisation
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many form ...
of α-alkenes and are notable for producing polyolefin
A polyolefin is a type of polymer with the general formula (CH2CHR)n where R is an alkyl group. They are usually derived from a small set of simple olefins (alkenes). Dominant in a commercial sense are polyethylene and polypropylene. More speciali ...
s with very high tacticities. Computational modelling
Computer simulation is the running of a mathematical model on a computer, the model being designed to represent the behaviour of, or the outcome of, a real-world or physical system. The reliability of some mathematical models can be determin ...
of the polymerisation mechanism indicates that kinetic factors likely account for the high tacticity.
Molecular recognition
Trisoxazolines baring a benzene backbone have been investigated formolecular recognition
Supramolecular chemistry refers to the branch of chemistry concerning Chemical species, chemical systems composed of a integer, discrete number of molecules. The strength of the forces responsible for spatial organization of the system range from w ...
and have shown promising selectivity for the recognition of ammonium
Ammonium is a modified form of ammonia that has an extra hydrogen atom. It is a positively charged (cationic) polyatomic ion, molecular ion with the chemical formula or . It is formed by the protonation, addition of a proton (a hydrogen nucleu ...
alkylammonium and sugar species, including examples of chiral recognition.
See also
*Bisoxazoline ligand
Bis(oxazoline) ligands (often abbreviated BOX ligands) are a class of chiral ligand, privileged chiral ligands containing two oxazoline rings. They are typically molecular symmetry, C2‑symmetric and exist in a wide variety of forms; with structur ...
s (BOX)
* Phosphinooxazolines
Phosphinooxazolines (often abbreviated PHOX) are a class of chiral ligands used in asymmetric catalysis. Colorless solids, PHOX ligands feature a tertiary phosphine group, often diphenyl, and an oxazoline ligand in the ortho position. The oxazolin ...
(PHOX)
* Trisoxazolinylborate
* Trispyrazolylborate
The trispyrazolylborate ligand, abbreviated Tp−, is an anionic Denticity, tridentate and tripodal ligand. Trispyrazolylborate refers specifically to the anion
B(C3N2H3)3
B, or b, is the second letter of the Latin alphabet, used in the modern English alphabet, the alphabets of other western European languages and others worldwide. Its name in English is ''bee'' (pronounced ), plural ''bees''.
It represent ...
ˆ’. However, the term can also be used to refer to derivatives having s ...References
{{reflist, 30em Coordination chemistry Oxazolines Tripodal ligands