Trioxidane on:
[Wikipedia]
[Google]
[Amazon]
Trioxidane (systematically named dihydrogen trioxide,), also called hydrogen trioxide is an 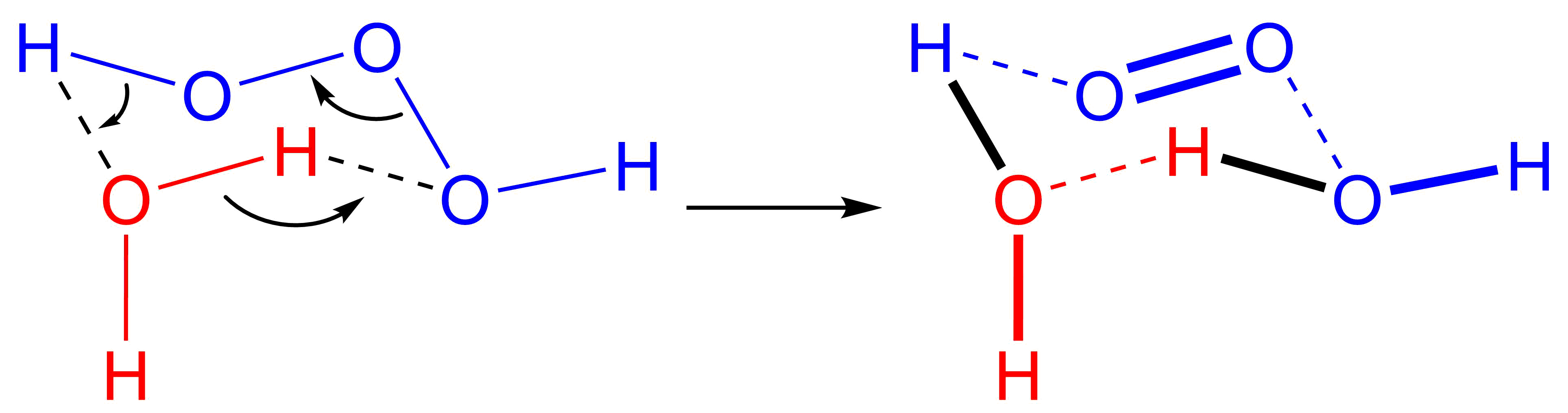 The reverse reaction, the addition of singlet oxygen to water, typically does not occur in part due to the scarcity of singlet oxygen. In biological systems, however,
The reverse reaction, the addition of singlet oxygen to water, typically does not occur in part due to the scarcity of singlet oxygen. In biological systems, however,
inorganic compound
An inorganic compound is typically a chemical compound that lacks carbon–hydrogen bondsthat is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as ''inorganic chemistry''.
Inorgan ...
with the chemical formula
A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as pare ...
(can be written as or ). It is one of the unstable hydrogen polyoxides. In aqueous solutions, trioxidane decomposes to form water and singlet oxygen
Singlet oxygen, systematically named dioxygen(singlet) and dioxidene, is a gaseous inorganic chemistry, inorganic chemical with the formula O=O (also written as or ), which is in a quantum state where all electrons are Radical (chemistry), spin p ...
:
 The reverse reaction, the addition of singlet oxygen to water, typically does not occur in part due to the scarcity of singlet oxygen. In biological systems, however,
The reverse reaction, the addition of singlet oxygen to water, typically does not occur in part due to the scarcity of singlet oxygen. In biological systems, however, ozone
Ozone () (or trioxygen) is an Inorganic compound, inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , break ...
is known to be generated from singlet oxygen, and the presumed mechanism is an antibody-catalyzed production of trioxidane from singlet oxygen.
Preparation
Trioxidane can be obtained in small, but detectable, amounts in reactions ofozone
Ozone () (or trioxygen) is an Inorganic compound, inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , break ...
and hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscosity, viscous than Properties of water, water. It is used as an oxidizer, bleaching agent, and antiseptic, usua ...
, or by the electrolysis of water
Electrolysis of water is using electricity to Water splitting, split water into oxygen () and hydrogen () gas by electrolysis. Hydrogen gas released in this way can be used as hydrogen fuel, but must be kept apart from the oxygen as the mixture ...
. Larger quantities have been prepared by the reaction of ozone with organic reducing agent
In chemistry, a reducing agent (also known as a reductant, reducer, or electron donor) is a chemical species that "donates" an electron to an (called the , , , or ).
Examples of substances that are common reducing agents include hydrogen, carbon ...
s at low temperatures in a variety of organic solvents, such as the anthraquinone process
The anthraquinone process, also called the Riedl–Pfleiderer process, is a process for the production of hydrogen peroxide, which was developed by IG Farben in the 1940s. The industrial production of hydrogen peroxide is based on the reduction o ...
. It is also formed during the decomposition of organic hydrotrioxides (ROOOH). Alternatively, trioxidane can be prepared by reduction of ozone with 1,2-diphenylhydrazine at low temperature. Using a resin-bound version of the latter, relatively pure trioxidane can be isolated as a solution in organic solvent. Preparation of high purity solutions is possible using the methyltrioxorhenium(VII) catalyst. In acetone-''d''6 at −20 °C, the characteristic 1H NMR signal of trioxidane could be observed at a chemical shift
In nuclear magnetic resonance (NMR) spectroscopy, the chemical shift is the resonant frequency of an atomic nucleus relative to a standard in a magnetic field. Often the position and number of chemical shifts are diagnostic of the structure of ...
of 13.1 ppm. Solutions of hydrogen trioxide in diethyl ether can be safely stored at −20 °C for as long as a week.
The reaction of ozone with hydrogen peroxide is known as the "peroxone process". This mixture has been used for some time for treating groundwater contaminated with organic compounds. The reaction produces H2O3 and H2O5.
Structure
In 1970–75, Giguère et al. observedinfrared
Infrared (IR; sometimes called infrared light) is electromagnetic radiation (EMR) with wavelengths longer than that of visible light but shorter than microwaves. The infrared spectral band begins with the waves that are just longer than those ...
and Raman spectra of dilute aqueous solutions of trioxidane. In 2005, trioxidane was observed experimentally by microwave spectroscopy in a supersonic jet. The molecule exists in a skewed structure, with an oxygen–oxygen–oxygen–hydrogen dihedral angle of 81.8°. The oxygen–oxygen bond length
In molecular geometry, bond length or bond distance is defined as the average distance between Atomic nucleus, nuclei of two chemical bond, bonded atoms in a molecule. It is a Transferability (chemistry), transferable property of a bond between at ...
s of 142.8 picometer
The picometre (international spelling as used by the International Bureau of Weights and Measures; SI symbol: pm) or picometer ( American spelling) is a unit of length in the International System of Units (SI), equal to , or one trillionth o ...
are slightly shorter than the 146.4 pm oxygen–oxygen bonds in hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscosity, viscous than Properties of water, water. It is used as an oxidizer, bleaching agent, and antiseptic, usua ...
. Various dimeric and trimeric forms also seem to exist.
There is a trend of increasing gas-phase acidity and corresponding p''K''a as the number of oxygen atoms in the chain increases in HO''n''H structures (''n''=1,2,3).
Reactions
Trioxidane readily decomposes into water and singlet oxygen, with a half-life of about 16 minutes in organic solvents at room temperature, but only milliseconds in water. It reacts with organic sulfides to formsulfoxide
In organic chemistry, a sulfoxide, also called a sulphoxide, is an organosulfur compound containing a sulfinyl () functional group attached to two carbon atoms. It is a polar functional group. Sulfoxides are oxidized derivatives of sulfides. E ...
s, but little else is known of its reactivity.
Recent research found that trioxidane is the active ingredient responsible for the antimicrobial
An antimicrobial is an agent that kills microorganisms (microbicide) or stops their growth (bacteriostatic agent). Antimicrobial medicines can be grouped according to the microorganisms they are used to treat. For example, antibiotics are used aga ...
properties of the well known ozone
Ozone () (or trioxygen) is an Inorganic compound, inorganic molecule with the chemical formula . It is a pale blue gas with a distinctively pungent smell. It is an allotrope of oxygen that is much less stable than the diatomic allotrope , break ...
/hydrogen peroxide
Hydrogen peroxide is a chemical compound with the formula . In its pure form, it is a very pale blue liquid that is slightly more viscosity, viscous than Properties of water, water. It is used as an oxidizer, bleaching agent, and antiseptic, usua ...
mix. Because these two compounds are present in biological systems as well it is argued that an antibody
An antibody (Ab) or immunoglobulin (Ig) is a large, Y-shaped protein belonging to the immunoglobulin superfamily which is used by the immune system to identify and neutralize antigens such as pathogenic bacteria, bacteria and viruses, includin ...
in the human body can generate trioxidane as a powerful oxidant
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or "Electron acceptor, accepts"/"receives" an electron from a (called the , , or ''electr ...
against invading bacteria. The source of the compound in biological systems is the reaction between singlet oxygen and water (which proceeds in either direction, of course, according to concentrations), with the singlet oxygen being produced by immune cells.
Computational chemistry
Computational chemistry is a branch of chemistry that uses computer simulations to assist in solving chemical problems. It uses methods of theoretical chemistry incorporated into computer programs to calculate the structures and properties of mol ...
predicts that more oxygen chain molecules or hydrogen polyoxides exist and that even indefinitely long oxygen chains can exist in a low-temperature gas. With this spectroscopic evidence a search for these types of molecules can start in interstellar space. A 2022 publication suggested the possibility of the presence of detectable concentrations of polyoxides in the atmosphere.
See also
* MolozonideReferences
{{Hydrides by group Inorganic compounds Oxides Polyoxides Oxoacids