Transition Metal Acyl Complexes on:
[Wikipedia]
[Google]
[Amazon]
 Transition metal acyl complexes describes
Transition metal acyl complexes describes
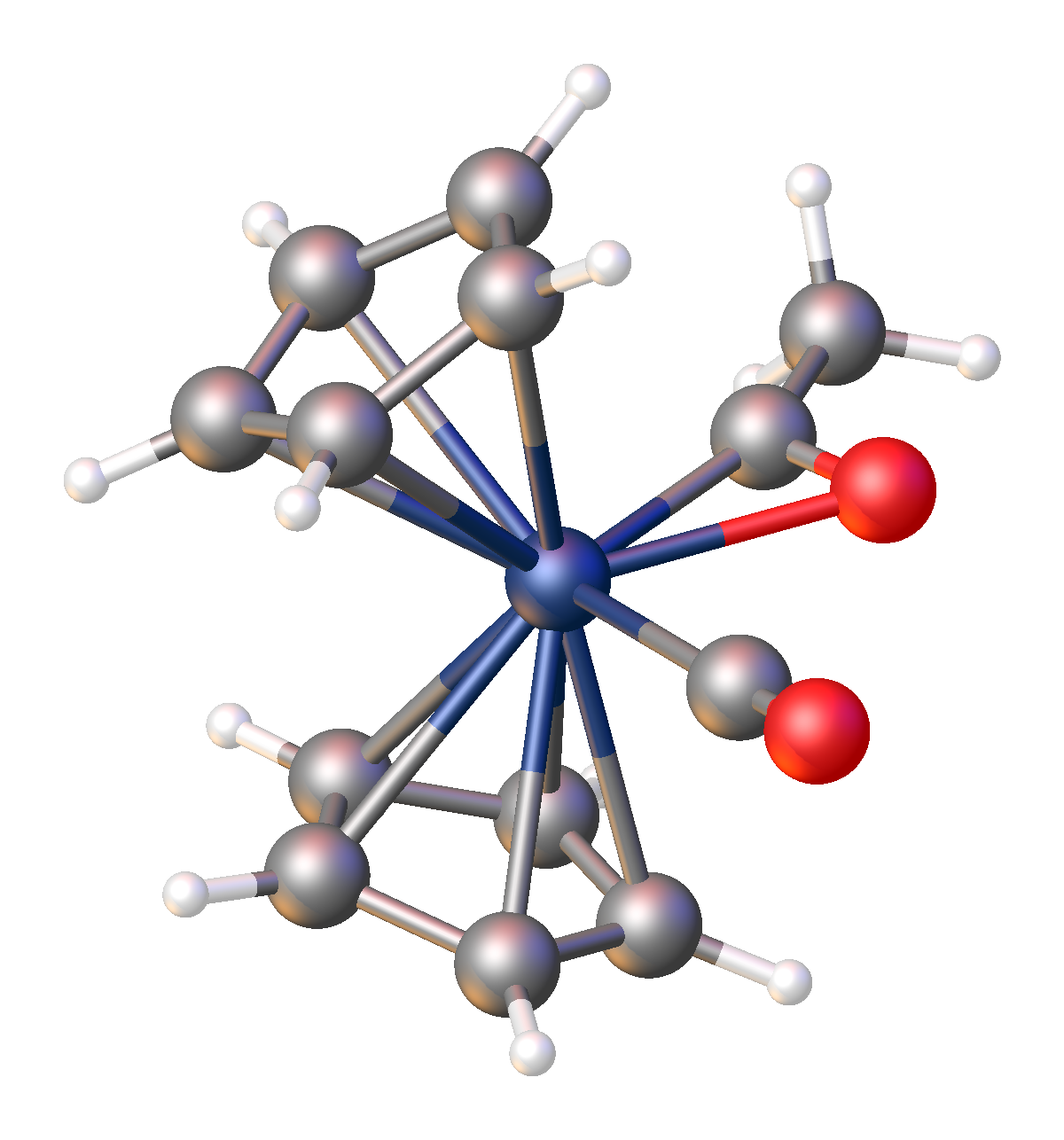 Acyl complexes are usually low-spin and spin-paired.
Monometallic acyl complexes adopt one of two related structures, C-bonded and η2-C-O-bonded. These forms sometimes interconvert. For the purpose of electron-counting, C-bonded acyl ligands count as 1-electron ligands, akin to pseudohalides. η2-Acyl ligands count as 3-electron "L-X" ligands.
bridging acyl ligands are also well known, where the carbon bonds to one metal and the oxygen bonds to a second metal. One example is the bis(μ-acetyl) complex CO)3Fe(C(O)CH3)2Fe(CO)3sup>2-.
Acyl complexes are usually low-spin and spin-paired.
Monometallic acyl complexes adopt one of two related structures, C-bonded and η2-C-O-bonded. These forms sometimes interconvert. For the purpose of electron-counting, C-bonded acyl ligands count as 1-electron ligands, akin to pseudohalides. η2-Acyl ligands count as 3-electron "L-X" ligands.
bridging acyl ligands are also well known, where the carbon bonds to one metal and the oxygen bonds to a second metal. One example is the bis(μ-acetyl) complex CO)3Fe(C(O)CH3)2Fe(CO)3sup>2-.
 Transition metal acyl complexes describes
Transition metal acyl complexes describes organometallic complex
Organometallic chemistry is the study of organometallic compounds, chemical compounds containing at least one chemical bond between a carbon atom of an organic molecule and a metal, including alkali, alkaline earth, and transition metals, ...
es containing one or more acyl (RCO) ligand
In coordination chemistry, a ligand is an ion or molecule ( functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's ele ...
s. Such compounds occur as transient intermediates in many industrially useful reactions, especially carbonylation
Carbonylation refers to reactions that introduce carbon monoxide into organic and inorganic substrates. Carbon monoxide is abundantly available and conveniently reactive, so it is widely used as a reactant in industrial chemistry. The term carbo ...
s.
Structure and bonding
 Acyl complexes are usually low-spin and spin-paired.
Monometallic acyl complexes adopt one of two related structures, C-bonded and η2-C-O-bonded. These forms sometimes interconvert. For the purpose of electron-counting, C-bonded acyl ligands count as 1-electron ligands, akin to pseudohalides. η2-Acyl ligands count as 3-electron "L-X" ligands.
bridging acyl ligands are also well known, where the carbon bonds to one metal and the oxygen bonds to a second metal. One example is the bis(μ-acetyl) complex CO)3Fe(C(O)CH3)2Fe(CO)3sup>2-.
Acyl complexes are usually low-spin and spin-paired.
Monometallic acyl complexes adopt one of two related structures, C-bonded and η2-C-O-bonded. These forms sometimes interconvert. For the purpose of electron-counting, C-bonded acyl ligands count as 1-electron ligands, akin to pseudohalides. η2-Acyl ligands count as 3-electron "L-X" ligands.
bridging acyl ligands are also well known, where the carbon bonds to one metal and the oxygen bonds to a second metal. One example is the bis(μ-acetyl) complex CO)3Fe(C(O)CH3)2Fe(CO)3sup>2-.
Synthesis
Metal acyls are often generated by the reaction of low-valent metal centers withacyl chloride
In organic chemistry, an acyl chloride (or acid chloride) is an organic compound with the functional group . Their formula is usually written , where R is a side chain. They are reactive derivatives of carboxylic acids (). A specific example ...
s. Illustrative is the oxidative addition
Oxidative addition and reductive elimination are two important and related classes of reactions in organometallic chemistry. Oxidative addition is a process that increases both the oxidation state and coordination number of a metal centre. Oxid ...
of acetyl chloride
Acetyl chloride (CH3COCl) is an acyl chloride derived from acetic acid. It belongs to the class of organic compounds called acid halides. It is a colorless, corrosive, volatile liquid. Its formula is commonly abbreviated to AcCl.
Synthesis
On ...
to Vaska's complex
Vaska's complex is the trivial name for the chemical compound ''trans''-carbonylchlorobis(triphenylphosphine)iridium(I), which has the formula IrCl(CO) (C6H5)3sub>2. This square planar diamagnetic organometallic complex consists of a central iri ...
, converting square planar Ir(I) to octahedral Ir(III):
:IrCl(CO)(PPh3)2 + CH3COCl → CH3COIrCl2(CO)(PPh3)2
In rare cases, acyls can be produced from aldehydes by C-H oxidative addition. This reaction underpins hydroacylation Hydroacylation is a type of organic reaction in which an alkene is inserted into the a formyl C-H bond. The product is a ketone. The reaction requires a metal catalyst. It is almost invariably practiced as an intramolecular reaction using homogeneou ...
.
In a related reaction, metal carbonyl anions are acylated by acyl chlorides:
:(C5H5)Fe(CO)2Na + CH3C(O)Cl → (C5H5)Fe(CO)2COCH3 + NaCl
Another important route to metal acyls entails insertion of CO into a metal alkyl bond. In this pathway, the alkyl ligand migrates to an adjacent CO ligand. This reaction is a step in the hydroformylation
Hydroformylation, also known as oxo synthesis or oxo process, is an industrial process for the production of aldehydes from alkenes. This chemical reaction entails the net addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon d ...
process.
Coordinatively saturated metal carbonyls react with organolithium reagents to give acyls. This reaction proceeds by attack of the alkyl nucleophile on the electrophilic CO ligand.
:
Reactions
In practical sense, the most important reaction of metal acyls is their detachment byreductive elimination
Reductive elimination is an elementary step in organometallic chemistry in which the oxidation state of the metal center decreases while forming a new covalent bond between two ligands. It is the microscopic reverse of oxidative addition, and ...
of aldehydes from acyl metal hydrides:
:LnMC(O)R(H) → LnM + RCHO
This reaction is the final step of hydroformylation
Hydroformylation, also known as oxo synthesis or oxo process, is an industrial process for the production of aldehydes from alkenes. This chemical reaction entails the net addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon d ...
.
Another important reaction is decarbonylation. This reaction requires that the acyl complex be coordinatively unsaturated:
:LnMC(O)R → Ln-1M(CO)R + L
:Ln-1MC(O)R → Ln-1M(CO)R
The oxygen center of acyl ligands is basic. This aspect is manifested in O-alkylations, which converts acyl complexes to alkoxycarbene complexes:
:
Applications
Metal acyl complexes participate in several commercial processes, including: *hydroformylation
Hydroformylation, also known as oxo synthesis or oxo process, is an industrial process for the production of aldehydes from alkenes. This chemical reaction entails the net addition of a formyl group (CHO) and a hydrogen atom to a carbon-carbon d ...
* acetic acid synthesis
*Eastman acetic anhydride process
*Ethylene-carbon monoxide copolymerization
A reaction involving metal acyl complexes of occasional value in organic synthesis is the Tsuji–Wilkinson decarbonylation reaction
The Tsuji–Wilkinson decarbonylation reaction is a method for the decarbonylation of aldehydes and some acyl chlorides. The reaction name recognizes , whose team first reported the use of Wilkinson's catalyst (RhCl(PPh3)3) for these reactions: ...
of aldehydes.
References