Transannular Strain on:
[Wikipedia]
[Google]
[Amazon]
 In
In
 By definition, strain implies discomfiture, so it should follow that molecules with large amounts of transannular strain should have higher energies than those without. Cyclohexane, for the most part, is without strain and is therefore quite stable and low in energy. Rings smaller than
By definition, strain implies discomfiture, so it should follow that molecules with large amounts of transannular strain should have higher energies than those without. Cyclohexane, for the most part, is without strain and is therefore quite stable and low in energy. Rings smaller than
 One specific example of a study of rates of reactions for an SN1 reaction is shown on the right. Various sized rings, ranging from four to seventeen members, were used to compare the relative rates and better understand the effect of transannular strain on this reaction. The solvolysis reaction in acetic acid involved the formation of a
One specific example of a study of rates of reactions for an SN1 reaction is shown on the right. Various sized rings, ranging from four to seventeen members, were used to compare the relative rates and better understand the effect of transannular strain on this reaction. The solvolysis reaction in acetic acid involved the formation of a

Link
* {{GoldBookRef, file=T06434, title=transannular strain Stereochemistry
 In
In organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms.Clay ...
, transannular strain (also called Prelog strain after chemist Vladimir Prelog) is the unfavorable interactions of ring substituents on non-adjacent carbons. These interactions, called transannular interactions, arise from a lack of space in the interior of the ring, which forces substituents into conflict with one another. In medium-sized cycloalkane
In organic chemistry, the cycloalkanes (also called naphthenes, but distinct from naphthalene) are the monocyclic saturated hydrocarbons. In other words, a cycloalkane consists only of hydrogen and carbon atoms arranged in a structure containin ...
s, which have between 8 and 11 carbons constituting the ring, transannular strain can be a major source of the overall strain, especially in some conformations, to which there is also contribution from large-angle strain and Pitzer strain.Smith and March, ''March's Advanced Organic Chemistry'', John Wiley & Sons Inc., 2007, In larger rings, transannular strain drops off until the ring is sufficiently large that it can adopt conformations devoid of any negative interactions.Anslyn and Dougherty, ''Modern Physical Organic Chemistry'', University Science Books, 2006,
Transannular strain can also be demonstrated in other cyclo-organic molecules, such as lactone
Lactones are cyclic carboxylic esters, containing a 1-oxacycloalkan-2-one structure (), or analogues having unsaturation or heteroatoms replacing one or more carbon atoms of the ring.
Lactones are formed by intramolecular esterification of the co ...
s, lactams, ether
In organic chemistry, ethers are a class of compounds that contain an ether group—an oxygen atom connected to two alkyl or aryl groups. They have the general formula , where R and R′ represent the alkyl or aryl groups. Ethers can again b ...
s, cycloalkenes, and cycloalkynes. These compounds are not without significance, since they are particularly useful in the study of transannular strain. Furthermore, transannular interactions are not relegated to only conflicts between hydrogen atoms, but can also arise from larger, more complicated substituents interacting across a ring.
Thermodynamics
 By definition, strain implies discomfiture, so it should follow that molecules with large amounts of transannular strain should have higher energies than those without. Cyclohexane, for the most part, is without strain and is therefore quite stable and low in energy. Rings smaller than
By definition, strain implies discomfiture, so it should follow that molecules with large amounts of transannular strain should have higher energies than those without. Cyclohexane, for the most part, is without strain and is therefore quite stable and low in energy. Rings smaller than cyclohexane
Cyclohexane is a cycloalkane with the molecular formula . Cyclohexane is non-polar. Cyclohexane is a colorless, flammable liquid with a distinctive detergent-like odor, reminiscent of cleaning products (in which it is sometimes used). Cyclohex ...
, like cyclopropane and cyclobutane
Cyclobutane is a cycloalkane and organic compound with the formula (CH2)4. Cyclobutane is a colourless gas and commercially available as a liquefied gas. Derivatives of cyclobutane are called cyclobutanes. Cyclobutane itself is of no commercia ...
, have significant tension caused by small-angle strain, but there is no transannular strain. While there is no small-angle strain present in medium-sized rings, there does exist something called large-angle strain. Some angle and torsional strain is used by rings with more than nine members to relieve some of the distress caused by transannular strain.
As the plot to the left indicates, the relative energies of cycloalkanes increases as the size of the ring increases, with a peak at cyclononane (with nine members in its ring.) At this point, the flexibility of the rings increases with increasing size; this allows for conformations that can significantly mitigate transannular interactions.
Kinetics
Rates of reaction can be affected by the size of rings. Essentially each reaction should be studied on a case-by-case basis but some general trends have been seen. Molecular mechanics calculations of strain energy differences SI between a sp2 and sp3 state in cycloalkanes show linear correlations with rates ( as logk ) of many reactions involving the transition between sp2 and sp3 states, such as ketone reduction, alcohol oxidation or nucleophilic substitution, the contribution of transannular strain is below 3 %. Rings with transannular strain have faster SN1, SN2, and free radical reactions compared to most smaller and normal sized rings. Five membered rings show an exception to this trend. On the other hand, somenucleophilic addition
In organic chemistry, a nucleophilic addition reaction is an addition reaction where a chemical compound with an electrophilic double or triple bond reacts with a nucleophile, such that the double or triple bond is broken. Nucleophilic additions ...
reactions involving addition to a carbonyl group in general show the opposite trend. Smaller and normal rings, with five membered rings being the anomaly, have faster reaction rates while those with transannular strain are slower.
 One specific example of a study of rates of reactions for an SN1 reaction is shown on the right. Various sized rings, ranging from four to seventeen members, were used to compare the relative rates and better understand the effect of transannular strain on this reaction. The solvolysis reaction in acetic acid involved the formation of a
One specific example of a study of rates of reactions for an SN1 reaction is shown on the right. Various sized rings, ranging from four to seventeen members, were used to compare the relative rates and better understand the effect of transannular strain on this reaction. The solvolysis reaction in acetic acid involved the formation of a carbocation
A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium , methanium and vinyl cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encoun ...
as the chloride ion leaves the cyclic molecule. This study fits the general trend seen above that rings with transannular strain show increased reactions rates compared to smaller rings in SN1 reactions.
Examples of transannular strain
Influence on regioselectivity
Theregioselectivity
In chemistry, regioselectivity is the preference of chemical bonding or breaking in one direction over all other possible directions. It can often apply to which of many possible positions a reagent will affect, such as which proton a strong base ...
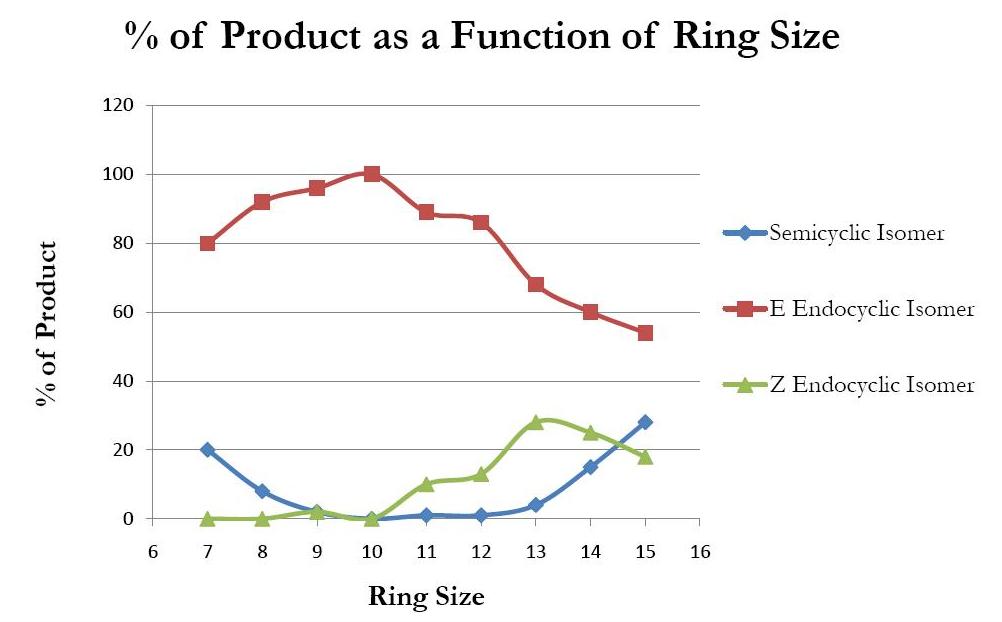
of water elimination is highly influenced by ring size. When water is eliminated from cyclic tertiary alcohols by an E1 route, three major products are formed. The semicyclic isomer (so-called because the double bond is shared by a ring atom and an exocyclic atom) and the (E) endocyclic isomer are expected to predominate; the (Z) endocyclic isomer is not expected to be formed until the ring size is large enough to accommodate the awkward angles of the trans configuration. The exact population of each product relative to the others differs considerably depending upon the size of the ring involved. As the ring size increases, the semicyclic isomer decreases rapidly and the (E) endocyclic isomer increases, but after a certain point, the semicyclic isomer begins to increase again. This can be attributed to transannular strain; this strain is significantly reduced in the (E) endocyclic isomer because it has one less substituent in the ring than the semicyclic isomer. 400x400px, Products yielded from the elimination of water from cyclic tertiary alcohols., thumb

Influence on medium-sized ring synthesis
One of the effects of transannular strain is the difficulty of synthesizing medium-sized rings. Illuminati et al. have studied thekinetics
Kinetics ( grc, κίνησις, , kinesis, ''movement'' or ''to move'') may refer to:
Science and medicine
* Kinetics (physics), the study of motion and its causes
** Rigid body kinetics, the study of the motion of rigid bodies
* Chemical k ...
of intramolecular ring closing using the simple nucleophilic substitution reaction of ortho-bromoalkoxyphenoxides. Specifically, they studied the ring closing of 5 to 10 carbon cyclic ethers. They found that as the number of carbons increased, so did the enthalpy
Enthalpy , a property of a thermodynamic system, is the sum of the system's internal energy and the product of its pressure and volume. It is a state function used in many measurements in chemical, biological, and physical systems at a constant ...
of activation for the reaction. This indicates that strain within the cyclic transition states is higher if there are more carbons in the ring. Since transannular strain is the largest source of strain in rings this size, the larger enthalpies of activation result in much slower cyclizations due to transannular interactions in the cyclic ethers.
Influence of bridges on transannular strain
Transannular strain can be eliminated by the simple addition of a carbon bridge. E,Z,E,Z,Z- 0annulene is quite unstable; while it has the requisite number of π-electrons to be aromatic, they are for the most part isolated. Ultimately, the molecule itself is very difficult to observe. However, by the simple addition of a methylene bridge between the 1 and 6 positions, a stable, flat, aromatic molecule can be made and observed.
References
External links
* Prelog strain definitionLink
* {{GoldBookRef, file=T06434, title=transannular strain Stereochemistry