Thiostrepton on:
[Wikipedia]
[Google]
[Amazon]
Thiostrepton is a natural cyclic



oligopeptide
An oligopeptide ('' oligo-'', "a few"), is a peptide consisting of two to twenty amino acids, including dipeptides, tripeptides, tetrapeptides, and other polypeptides. Some of the major classes of naturally occurring oligopeptides include aerugi ...
antibiotic
An antibiotic is a type of antimicrobial substance active against bacteria. It is the most important type of antibacterial agent for fighting pathogenic bacteria, bacterial infections, and antibiotic medications are widely used in the therapy ...
of the thiopeptide class, derived from several strains of streptomycetes, such as '' Streptomyces azureus'' and '' Streptomyces laurentii''. Thiostrepton is a natural product
A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical s ...
of the ribosomally synthesized and post-translationally modified peptide (RiPP) class.
History
Thiostrepton was discovered by Donovick ''et al.'' who described its antibacterial properties in 1955.Dorothy Crowfoot Hodgkin
Dorothy Mary Crowfoot Hodgkin (née Crowfoot; 12 May 1910 – 29 July 1994) was a Nobel Prize-winning English chemist who advanced the technique of X-ray crystallography to determine the structure of biomolecules, which became essential for ...
solved the structure of thiostrepton in 1970.
Early in 1978, Bycroft and Gowland
proposed the biosynthesis of thiostrepton, which was still unclear until 2009. Several studies of thiopeptide biosynthesis have been contemporarily published in 2009 and two of them (Liao ''et al.'' and Kelly ''et al.'') included the similar biosynthesis of thiostrepton: it's ribosomally synthesized from thiostrepton biosynthetic genes (tsr genes) and posttranslational modification is needed.
A total synthesis of thiostrepton was completed by K.C. Nicolaou, ''et al.'' in 2004.
Applications
Thiostrepton has been used in veterinary medicine inmastitis
Mastitis is inflammation of the breast or udder, usually associated with breastfeeding. Symptoms typically include local pain and redness. There is often an associated fever and general soreness. Onset is typically fairly rapid and usually occ ...
caused by gram-negative
Gram-negative bacteria are bacteria that, unlike gram-positive bacteria, do not retain the crystal violet stain used in the Gram staining method of bacterial differentiation. Their defining characteristic is that their cell envelope consists ...
organisms and in dermatologic disorders. It is mostly used in complex ointments containing neomycin
Neomycin, also known as framycetin, is an aminoglycoside antibiotic that displays bactericidal activity against Gram-negative aerobic bacilli and some anaerobic bacilli where resistance has not yet arisen. It is generally not effective against ...
, nystatin
Nystatin, sold under the brand name Mycostatin among others, is an antifungal medication. It is used to treat ''Candida (fungus), Candida'' infections of the skin including diaper rash, Candidiasis, thrush, esophageal candidiasis, and vaginal ...
, Thiostrepton and topical steroid
A steroid is an organic compound with four fused compound, fused rings (designated A, B, C, and D) arranged in a specific molecular configuration.
Steroids have two principal biological functions: as important components of cell membranes t ...
s. It is also active against gram-positive bacteria. It is notable that ointments for human usage contain neomycin
Neomycin, also known as framycetin, is an aminoglycoside antibiotic that displays bactericidal activity against Gram-negative aerobic bacilli and some anaerobic bacilli where resistance has not yet arisen. It is generally not effective against ...
, nystatin
Nystatin, sold under the brand name Mycostatin among others, is an antifungal medication. It is used to treat ''Candida (fungus), Candida'' infections of the skin including diaper rash, Candidiasis, thrush, esophageal candidiasis, and vaginal ...
, and topical steroid
A steroid is an organic compound with four fused compound, fused rings (designated A, B, C, and D) arranged in a specific molecular configuration.
Steroids have two principal biological functions: as important components of cell membranes t ...
s, but no thiostrepton.
Thiostrepton was reported (in 2008) to exhibit activity against breast cancer cells through targeting the transcription factor forkhead box M1 (FOXM1
Forkhead box protein M1 is a protein that in humans is encoded by the ''FOXM1'' gene. The protein encoded by this gene is a member of the FOX family of transcription factors. Its potential as a target for future cancer treatments led to it being ...
), also in 2011.
It has also been shown to circumvent acquired cisplatin resistance in breast cancer cells under ''in vitro'' conditions.
Thiostrepton is used in molecular biology as a reagent for both positive and negative selection of genes involved in nucleotide metabolism.
Thiostrepton has also shown promise in treating osteoporosis in animal models because it can inhibit unusual osteoclast precursor cells.
The antibiotic thiostrepton was identified as an insulin resistance
Insulin resistance (IR) is a pathological response in which cells in insulin-sensitive tissues in the body fail to respond normally to the hormone insulin or downregulate insulin receptors in response to hyperinsulinemia.
Insulin is a horm ...
reversal agent. Subsequent validation in ''ex vivo'' insulin-resistant mouse muscle and palmitate-induced insulin-resistant myotubes demonstrated potent insulin action restoration, possibly via upregulation of glycolysis due to attenuation of mitochondrial oxidative phosphorylation by thiostrepton.
Effects of thiostrepton on stringent response
Thiostrepton binds to the L11 protein of the large ribosomal subunit. Mutations in this protein confer resistance to this metabolite. The L11 protein is also essential for regulating the RelA protein and activating the synthesis of the cellular alarmone (p)ppGpp, which, in turn, triggers the stringent response and antibiotic synthesis. Furthermore, amino acid substitutions in the L11 protein that confer resistance to thiostrepton also inhibit the stringent response in strains belonging to the ''Streptomyces'' genus. A similar mechanism has been observed in ''Neisseria gonorrhoeae'', where thiostrepton reduces the synthesis of (p)ppGpp, inhibiting the activation of the persistence. Persistence is a mechanism that allows bacteria to survive antibiotic treatments and other stressors by enabling a subpopulation of bacteria to become metabolically inactive.Biosynthesis
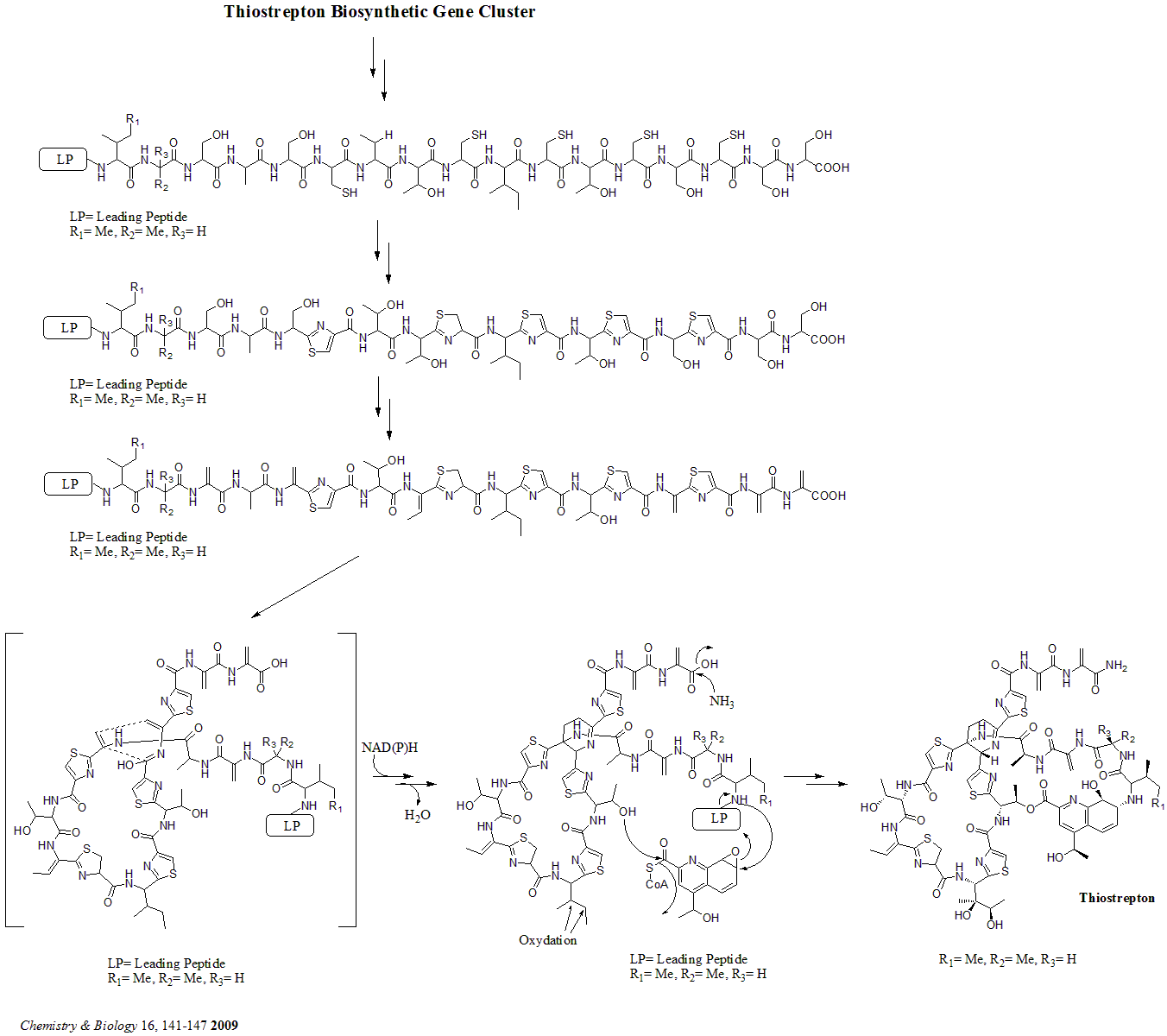
There are total 21 genes (tsrA~tsrU) in the biosynthetic gene cluster. The precursor of thiostrepton contains 58 amino acids in the peptide chain, which includes 41-aa leader peptide (LP) and 17-aa structural peptide (IASASCTTCICTCSCSS). Once the precursor is synthesized, cyclodehydratase tsrO and dehydrogenase tsrM catalyze the formation of thiazole or thiazoline from every cysteine residues in the peptide chain. After thiazole/thiazoline formation, dehydratases tsrJ, K and S then convert all the serine residues into dehydroalanines. A hetero Diels-Alder cyclization of the central dehydropiperidine (at S5, C13, and S14) has been suggested by Bycroft back to 1978 and been employed in the chemical synthesis of this core structure by Nicolaou ''et al.'' in 2005. An alternative mechanism of the dehydropiperidine formation has also been suggested by Kelly ''et al''. in 2009. Nevertheless, based on experimental evidence, tsrN and L are suggested to be responsible for the hetero Diels-Alder cyclization. The quinaldic acid moiety is suggested to be synthesized by the nine genes tsrFAEBDUPQI from tryptophan and then results in the closure of quinaldic acid macrocycle. At last, tsrR serves as a candidate for the oxidation of the Ile residue to afford thiostrepton.
Alternative mechanism for the formation of the dehydropiperidine core

Total synthesis
In 2005, Nicolaou ''et al.'' published the total synthesis of thiostrepton. At first, they constructed the key building blocks of thiostrepton (1): dehydropiperidine core (2), thiazoline macrocycle (3), bis-dehydroalanine tail (4), and quinaldic acid macrocycle (5). Then they assembled the building blocks sequentially as shown in the synthetic scheme (compound numbers are from the reference).Building blocks

Synthetic scheme

References
{{reflist, 33em Thiopeptides Heterocyclic compounds with 7 or more rings Antibiotics