Tetrafluoroboric Acid on:
[Wikipedia]
[Google]
[Amazon]
Fluoroboric acid or tetrafluoroboric acid (archaically, fluoboric acid) is an
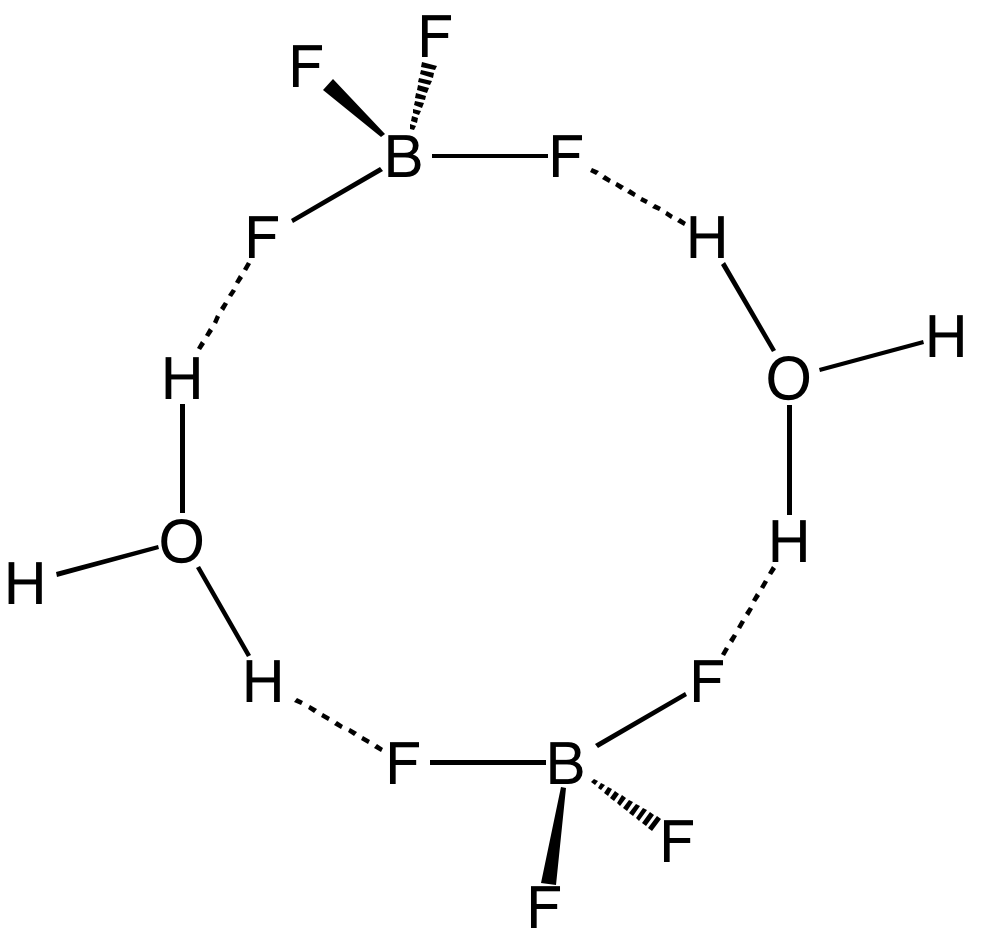 Aqueous solutions of are produced by dissolving
Aqueous solutions of are produced by dissolving
inorganic compound
An inorganic compound is typically a chemical compound that lacks carbon–hydrogen bondsthat is, a compound that is not an organic compound. The study of inorganic compounds is a subfield of chemistry known as ''inorganic chemistry''.
Inorgan ...
with the simplified chemical formula
A chemical formula is a way of presenting information about the chemical proportions of atoms that constitute a particular chemical compound or molecule, using chemical element symbols, numbers, and sometimes also other symbols, such as pare ...
. Solvent-free tetrafluoroboric acid () has not been reported. The term "fluoroboric acid" usually refers to a range of compounds including hydronium
In chemistry, hydronium (hydroxonium in traditional British English) is the cation , also written as , the type of oxonium ion produced by protonation of water. It is often viewed as the positive ion present when an Arrhenius acid is dissolved ...
tetrafluoroborate (), which are available as solutions. The ethyl ether
Diethyl ether, or simply ether, is an organic compound with the chemical formula , sometimes abbreviated as . It is a colourless, highly volatile, sweet-smelling ("ethereal odour"), extremely flammable liquid. It belongs to the ether class of o ...
solvate is also commercially available, where the fluoroboric acid can be represented by the formula , where ''n'' is 2.
It is mainly produced as a precursor to other fluoroborate salts.Gregory K. Friestad, Bruce P. Branchaud "Tetrafluoroboric Acid" E-Eros Encyclopedia of Reagents for Organic Synthesis. It is a strong acid
An acid is a molecule or ion capable of either donating a proton (i.e. Hydron, hydrogen cation, H+), known as a Brønsted–Lowry acid–base theory, Brønsted–Lowry acid, or forming a covalent bond with an electron pair, known as a Lewis ...
. Fluoroboric acid is corrosive and attacks the skin. It is available commercially as a solution in water and other solvent
A solvent (from the Latin language, Latin ''wikt:solvo#Latin, solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a Solution (chemistry), solution. A solvent is usually a liquid but can also be a solid, a gas ...
s such as diethyl ether
Diethyl ether, or simply ether, is an organic compound with the chemical formula , sometimes abbreviated as . It is a colourless, highly Volatility (chemistry), volatile, sweet-smelling ("ethereal odour"), extremely flammable liquid. It belongs ...
. It is a strong acid with a weakly coordinating, non-oxidizing conjugate base. It is structurally similar to perchloric acid, but lacks the hazards associated with oxidant
An oxidizing agent (also known as an oxidant, oxidizer, electron recipient, or electron acceptor) is a substance in a redox chemical reaction that gains or "Electron acceptor, accepts"/"receives" an electron from a (called the , , or ''electr ...
s.
Structure and production
Pure has not been described. The same holds true for the superacids that are known by the simplified formulas and . However, a solution of in HF is highly acidic, having an approximate speciation of (fluoronium tetrafluoroborate) and a Hammett acidity function of −16.6 at 7 mol % , easily qualifying as a superacid. Although the solvent-free has not been isolated, its solvates are well characterized. These salts consist of protonated solvent as a cation, e.g., and , and the tetrahedral anion. The anion and cations are strongly hydrogen-bonded. Aqueous solutions of are produced by dissolving
Aqueous solutions of are produced by dissolving boric acid
Boric acid, more specifically orthoboric acid, is a compound of boron, oxygen, and hydrogen with formula . It may also be called hydrogen orthoborate, trihydroxidoboron or boracic acid. It is usually encountered as colorless crystals or a white ...
in aqueous hydrofluoric acid
Hydrofluoric acid is a solution of hydrogen fluoride (HF) in water. Solutions of HF are colorless, acidic and highly corrosive. A common concentration is 49% (48–52%) but there are also stronger solutions (e.g. 70%) and pure HF has a boiling p ...
. Three equivalents of HF react to give the intermediate boron trifluoride and the fourth gives fluoroboric acid:
:
An anhydrous fluoroboric acid solution can be prepared by adding aqueous fluoroboric acid to an excess of acetic anhydride
Acetic anhydride, or ethanoic anhydride, is the chemical compound with the chemical formula, formula . Commonly abbreviated , it is one the simplest organic acid anhydride, anhydrides of a carboxylic acid and is widely used in the production of c ...
at 0°C, which produces a solution of fluoroboric acid, acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main compone ...
, and residual acetic anhydride.
Acidity
The acidity of fluoroboric acid is complicated by the fact that its name refers to a range of different compounds, e.g. (dimethyloxonium tetrafluoroborate), (oxonium tetrafluoroborate), and (hydrogen fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluori ...
- boron trifluoride 1:1 adduct) – each with a different acidity. The aqueous p''K''a is quoted as −0.44. Titration
Titration (also known as titrimetry and volumetric analysis) is a common laboratory method of Quantitative research, quantitative Analytical chemistry, chemical analysis to determine the concentration of an identified analyte (a substance to be ...
of (tetrabutylammonium tetrafluoroborate) in acetonitrile
Acetonitrile, often abbreviated MeCN (methyl cyanide), is the chemical compound with the formula and structure . This colourless liquid is the simplest organic nitrile (hydrogen cyanide is a simpler nitrile, but the cyanide anion is not class ...
solution indicates that , i.e., , has a p''K''a of 1.6 in that solvent. Its acidity is thus comparable to that of fluorosulfonic acid
Fluorosulfuric acid (IUPAC name: sulfurofluoridic acid) is the inorganic compound with the chemical formula . It is one of the strongest acids commercially available. It is a tetrahedral molecule and is closely related to sulfuric acid, , substitu ...
.
Applications
Fluoroboric acid is the principal precursor to fluoroborate salts, which are typically prepared by treating the metal oxides with fluoroboric acid. The inorganic salts are intermediates in the manufacture of flame-retardant materials and glazing frits, and in electrolytic generation ofboron
Boron is a chemical element; it has symbol B and atomic number 5. In its crystalline form it is a brittle, dark, lustrous metalloid; in its amorphous form it is a brown powder. As the lightest element of the boron group it has three ...
. is also used in aluminum etching and acid pickling.
Organic chemistry
is used as acatalyst
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
for alkylation Alkylation is a chemical reaction that entails transfer of an alkyl group. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecting al ...
s and polymerizations. In carbohydrate protection reactions, ethereal fluoroboric acid is an efficient and cost-effective catalyst for transacetalation and isopropylidenation reactions. Acetonitrile
Acetonitrile, often abbreviated MeCN (methyl cyanide), is the chemical compound with the formula and structure . This colourless liquid is the simplest organic nitrile (hydrogen cyanide is a simpler nitrile, but the cyanide anion is not class ...
solutions cleave acetals and some ethers. Many reactive cations have been obtained using fluoroboric acid, e.g. tropylium tetrafluoroborate (), triphenylcarbenium tetrafluoroborate (), triethyloxonium tetrafluoroborate (), and benzenediazonium tetrafluoroborate ().
Electroplating
Solutions of are used in the electroplating of tin and tin alloys. In this application, methanesulfonic acid is displacing the use of . Fluoroboric acid is also used for high-speed electroplating of copper in fluoroborate baths.Safety
Fluoroboric acid is toxic and attacks skin and eyes. It attacks glass. It hydrolyzes, releasing corrosive, volatilehydrogen fluoride
Hydrogen fluoride (fluorane) is an Inorganic chemistry, inorganic compound with chemical formula . It is a very poisonous, colorless gas or liquid that dissolves in water to yield hydrofluoric acid. It is the principal industrial source of fluori ...
.
Other fluoroboric acids
A series of fluoroboric acids is known in aqueous solutions. The series can be presented as follows: * (hydrogen tetrahydroxyborate) (not a fluoroboric acid) * (hydrogen fluoro(trihydroxy)borate) * (hydrogen difluoro(dihydroxy)borate) * (hydrogen trifluoro(hydroxy)borate) * (hydrogen tetrafluoroborate)See also
* Fluorosulfuric acid * Fluoroantimonic acidReferences
Further reading
* * * * * * * *External links
* {{fluorine compounds Tetrafluoroborates Mineral acids