Strontian Process on:
[Wikipedia]
[Google]
[Amazon]
The strontian process is an obsolete chemical method to recover sugar from
 Strontium carbonate is a recycled coreactant in this process.
# Strontium carbonate is
Strontium carbonate is a recycled coreactant in this process.
# Strontium carbonate is
(PDF; 4,3 MB)
''(German).'' One of the biggest mines, at
(PDF; 6,5 MB)
''In German.'' * Heriot, T. H. P.: ''The Manufacture of Sugar from the Cane and Beet'', Green and Company, 1920, pp. 341–34
(archive online)
* ''Krause, G.: ''Der Schiedsspruch in Sachen des Scheibler'schen Monostrontiumsaccharat-Patentes'', Chemiker Zeitung, nr. 32, 19th April, 1885
(PDF; 4,94 MB)
''In German.''
molasses
Molasses () is a viscous byproduct, principally obtained from the refining of sugarcane or sugar beet juice into sugar. Molasses varies in the amount of sugar, the method of extraction, and the age of the plant. Sugarcane molasses is usuall ...
. Its use in Europe peaked in the middle of the 19th century. The name ''strontian'' comes from the Scottish village Strontian
Strontian (;
) is the main village in Sunart, an area in western Lochaber, Scottish Highlands, Highland, Scotland, on the A861 road. Prior to 1975 it was part of Argyllshire. It lies on the north shore of Loch Sunart, close to the head of th ...
where the source mineral strontianite
Strontianite (Strontium, SrCarbon, COxygen, O3) is an important raw material for the extraction of strontium. It is a rare carbonate mineral and one of only a few strontium minerals. It is a member of the aragonite group.
Aragonite group membe ...
(strontium carbonate
Strontium carbonate (SrCO3) is the carbonate salt of strontium that has the appearance of a white or grey powder. It occurs in nature as the mineral strontianite.
Chemical properties
Strontium carbonate is a white, odorless, tasteless powder ...
) was first found.
Chemistry
 Strontium carbonate is a recycled coreactant in this process.
# Strontium carbonate is
Strontium carbonate is a recycled coreactant in this process.
# Strontium carbonate is calcined
Calcination is thermal treatment of a solid chemical compound (e.g. mixed carbonate ores) whereby the compound is raised to high temperature without melting under restricted supply of ambient oxygen (i.e. gaseous O2 fraction of air), generally fo ...
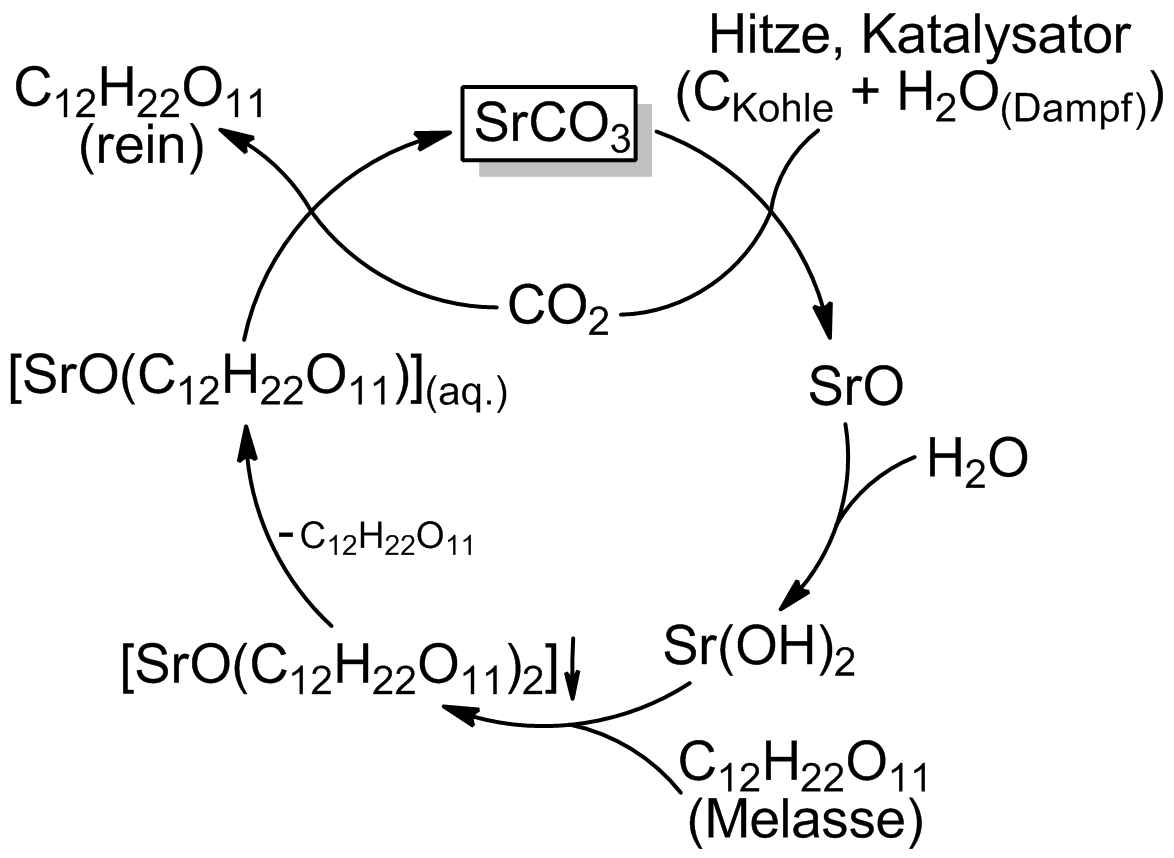
with carbon in the presence of steam to form strontium hydroxide. The strontium and carbon dioxide formed are rejoined later in the process, forming strontium carbonate once again.
#: SrCO3 + C + H2O + O2 = Sr(OH)2 + 2 CO2
# In a molasses solution kept near 100 °C, the hydroxide reacts with soluble sugars to form water and the poorly soluble strontium saccharide which is filtered out, but kept awash in near-boiling water.
#: Sr(OH)2 + 2C12H22O11 = SrO(C12H22O11)2 + H2O
# The saccharate liquid is cooled to 10 °C, cracking off one of the sugars
#: SrO(C12H22O11)2 = SrO(C12H22O11) + C12H22O11
# The carbon dioxide (from the calcination) is bubbled through the saccharate solution, cracking off the second sugar and reforming the strontium carbonate, which is filtered off.
#: SrO(C12H22O11) + CO2 = SrCO3 + C12H22O11
# The sugar is then extracted through evaporating the remaining solution.
There are two types of strontium saccharide
A carbohydrate () is a biomolecule composed of carbon (C), hydrogen (H), and oxygen (O) atoms. The typical hydrogen-to-oxygen atomic ratio is 2:1, analogous to that of water, and is represented by the empirical formula (where ''m'' and ''n'' m ...
: one at low temperature, the strontium monosaccharide
Monosaccharides (from Greek '' monos'': single, '' sacchar'': sugar), also called simple sugars, are the simplest forms of sugar and the most basic units (monomers) from which all carbohydrates are built.
Chemically, monosaccharides are polyhy ...
; and the second at high temperature, the strontium disaccharide
A disaccharide (also called a double sugar or ''biose'') is the sugar formed when two monosaccharides are joined by glycosidic linkage. Like monosaccharides, disaccharides are simple sugars soluble in water. Three common examples are sucrose, ...
.
History
Molasses is the first stage output of several differentsugar production
The sugar industry subsumes the production, processing and marketing of sugars (mostly sucrose and fructose). Globally, about 80% of sugar is extracted from sugar cane, grown predominantly in the tropics, and 20% from sugar beet, grown mostly in ...
processes, and contains more than 50% sugar. The French chemists Hippolyte Leplay and Augustin-Pierre Dubrunfaut
Augustin-Pierre Dubrunfaut (; Lille, 1 September 1797 – Paris, 7 October 1881) was a French chemist.
Mutarotation was discovered by Dubrunfaut in 1844, when he noticed that the specific rotation of aqueous sugar solution changes with time. In ...
developed a process for extracting sugar from molasses, reacting them with barium oxide
Barium oxide, also known as baria, is a white hygroscopic non-flammable chemical compound, compound with the formula BaO. It has a Cubic crystal system, cubic structure and is used in cathode-ray tubes, crown glass, and Catalysis, catalysts. It ...
, to give the insoluble barium-saccharates. In 1849, they expanded their patent to include strontium salts. Apparently, this patent application had the only purpose to legally secure the so-called ''baryte process'', since the strontian process from Leplay and Dubrunfaut probably wouldn't work as described.
Only later, through the work of Carl Scheibler (patents dated 1881, 1882, and 1883), was it possible to apply the strontian process on an industrial basis.
According to Scheibler the procedure must be carried out at boiling temperatures.
Repercussion in Germany
The Scheibler procedure came into use in the Dessauer Sugar Refinery (inDessau
Dessau is a district of the independent city of Dessau-Roßlau in Saxony-Anhalt at the confluence of the rivers Mulde and Elbe, in the ''States of Germany, Bundesland'' (Federal State) of Saxony-Anhalt. Until 1 July 2007, it was an independent ...
), through Emil Fleischer. In the Münsterland region, its arrival caused a ″gold fever″ breakout, regarding the strontianite
Strontianite (Strontium, SrCarbon, COxygen, O3) is an important raw material for the extraction of strontium. It is a rare carbonate mineral and one of only a few strontium minerals. It is a member of the aragonite group.
Aragonite group membe ...
mining.Martin Börnchen: ''Der Strontianitbergbau im Münsterland'(PDF; 4,3 MB)
''(German).'' One of the biggest mines, at
Drensteinfurt
Drensteinfurt (in low German ''Stewwert'') is a town in the district of Warendorf, in North Rhine-Westphalia, Germany. It is situated approximately 15 km north of Hamm and 20 km south of Münster. The villages Rinkerode in the north a ...
, was named after Dr. Reichardt, the director of the Dessauer Sugar Refinery. A further place the strontian process came to be used was the Sugar Factory Rositz (in Rositz).
Yet by 1883, the demand for strontianite had begun to shrink. First, it was replaced by another strontium mineral (celestine Celestine is a given name and a surname.
People Given name
* Pope Celestine I (died 432)
* Pope Celestine II (died 1144)
* Pope Celestine III (c. 1106–1198)
* Pope Celestine IV (died 1241)
* Pope Celestine V (1215–1296)
* Antipope Cel ...
), that could be imported from England, in a cheaper way. Second, the prices for sugar decreased so much, that the production from molasses was no longer worthwhile.
Literature (further reading)
* Börnchen, Martin : ''Strontianit'', Exhibition guide from the University Library of theFree University of Berlin
The Free University of Berlin (, often abbreviated as FU Berlin or simply FU) is a public university, public research university in Berlin, Germany. It was founded in West Berlin in 1948 with American support during the early Cold War period a ...
, 2005''(PDF; 6,5 MB)
''In German.'' * Heriot, T. H. P.: ''The Manufacture of Sugar from the Cane and Beet'', Green and Company, 1920, pp. 341–34
(archive online)
* ''Krause, G.: ''Der Schiedsspruch in Sachen des Scheibler'schen Monostrontiumsaccharat-Patentes'', Chemiker Zeitung, nr. 32, 19th April, 1885
(PDF; 4,94 MB)
''In German.''
References
{{reflist Chemical processes Industrial processes Catalysis Strontium Strontium minerals History of sugar Sugar production