Stone–Wales Defect on:
[Wikipedia]
[Google]
[Amazon]
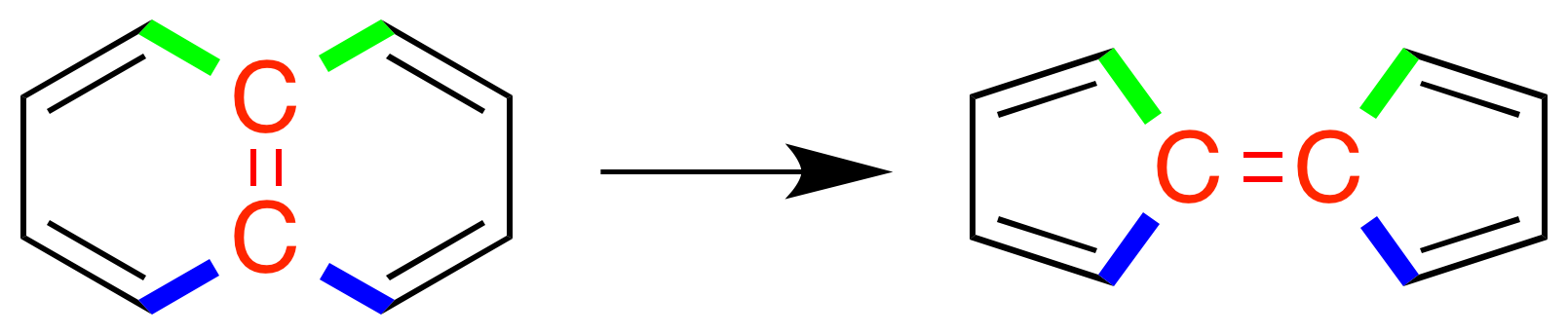 A Stone–Wales defect is a
A Stone–Wales defect is a  The reaction occurs on
The reaction occurs on
 A Stone–Wales defect is a
A Stone–Wales defect is a crystallographic defect
A crystallographic defect is an interruption of the regular patterns of arrangement of atoms or molecules in Crystal, crystalline solids. The positions and orientations of particles, which are repeating at fixed distances determined by the Crysta ...
that involves the change of connectivity of two π-bonded carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
atoms, leading to their rotation by 90° with respect to the midpoint of their bond. The reaction commonly involves conversion between a naphthalene
Naphthalene is an organic compound with formula . It is the simplest polycyclic aromatic hydrocarbon, and is a white Crystal, crystalline solid with a characteristic odor that is detectable at concentrations as low as 0.08 Parts-per notation ...
-like structure into a fulvalene
Fulvalene (bicyclopentadienylidene) is the member of the fulvalene family with the molecular formula C10H8. It is of theoretical interest as one of the simplest non-benzenoid conjugated hydrocarbons. Fulvalene is an unstable isomer of the mor ...
-like structure, that is, two rings that share an edge vs two separate rings that have vertices bonded to each other.
 The reaction occurs on
The reaction occurs on carbon nanotube
A carbon nanotube (CNT) is a tube made of carbon with a diameter in the nanometre range ( nanoscale). They are one of the allotropes of carbon. Two broad classes of carbon nanotubes are recognized:
* ''Single-walled carbon nanotubes'' (''S ...
s, graphene
Graphene () is a carbon allotrope consisting of a Single-layer materials, single layer of atoms arranged in a hexagonal lattice, honeycomb planar nanostructure. The name "graphene" is derived from "graphite" and the suffix -ene, indicating ...
, and similar carbon frameworks, where the four adjacent six-membered rings of a pyrene
Pyrene is a polycyclic aromatic hydrocarbon (PAH) consisting of four fused benzene rings, resulting in a flat aromatic system. The chemical formula is . This yellow-green solid is the smallest peri-fused PAH (one where the rings are fused thro ...
-like region are changed into two five-membered rings and two seven-membered rings when the bond uniting two of the adjacent rings rotates. In these materials, the rearrangement is thought to have important implications for the thermal, chemical, electrical, and mechanical properties. The rearrangement is an example of a pyracyclene rearrangement.
History
The defect is named after Anthony Stone andDavid J. Wales
David John Wales One or more of the preceding sentences incorporates text from the royalsociety.org website where: (born 1963) is a professor of chemical physics in the Department of Chemistry at the University of Cambridge.
Education
Wales ...
at the University of Cambridge
The University of Cambridge is a Public university, public collegiate university, collegiate research university in Cambridge, England. Founded in 1209, the University of Cambridge is the List of oldest universities in continuous operation, wo ...
, who described it in a 1986 paper on the isomerization
In chemistry, isomerization or isomerisation is the process in which a molecule, polyatomic ion or molecular fragment is transformed into an isomer with a different chemical structure. Enolization is an example of isomerization, as is tautomer ...
of fullerenes
A fullerene is an allotrope of carbon whose molecules consist of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to six atoms. The molecules may have hollow sphere- ...
. However, a similar defect was described much earlier by G. J. Dienes in 1952 in a paper on diffusion mechanisms in graphite
Graphite () is a Crystallinity, crystalline allotrope (form) of the element carbon. It consists of many stacked Layered materials, layers of graphene, typically in excess of hundreds of layers. Graphite occurs naturally and is the most stable ...
and later in 1969 in a paper on defects in graphite
Graphite () is a Crystallinity, crystalline allotrope (form) of the element carbon. It consists of many stacked Layered materials, layers of graphene, typically in excess of hundreds of layers. Graphite occurs naturally and is the most stable ...
by Peter Thrower. For this reason, the term Stone–Thrower–Wales defect is sometimes used.
Structural effects
The defects have been imaged usingscanning tunneling microscopy
A scanning tunneling microscope (STM) is a type of scanning probe microscope used for imaging surfaces at the atomic level. Its development in 1981 earned its inventors, Gerd Binnig and Heinrich Rohrer, then at IBM Zürich, the Nobel Prize in ...
and transmission electron microscopy
Transmission electron microscopy (TEM) is a microscopy technique in which a beam of electrons is transmitted through a specimen to form an image. The specimen is most often an ultrathin section less than 100 nm thick or a suspension on a g ...
and can be determined using various vibrational spectroscopy
Infrared spectroscopy (IR spectroscopy or vibrational spectroscopy) is the measurement of the interaction of infrared radiation with matter by absorption, emission, or reflection. It is used to study and identify chemical substances or functio ...
techniques.
It has been proposed that the coalescence
Coalesce, coalescence or coalescent can refer to:
Chemistry and physics
* Coalescence (chemistry), the process by which two or more separate masses of miscible substances seem to "pull" each other together should they make the slightest contac ...
process of fullerene
A fullerene is an allotropes of carbon, allotrope of carbon whose molecules consist of carbon atoms connected by single and double bonds so as to form a closed or partially closed mesh, with fused rings of five to six atoms. The molecules may ...
s or carbon nanotubes may occur through a sequence of such a rearrangements. The defect is thought to be responsible for nanoscale plasticity
Plasticity may refer to:
Science
* Plasticity (physics), in engineering and physics, the propensity of a solid material to undergo permanent deformation under load
* Behavioral plasticity, change in an organism's behavior in response to exposur ...
and the brittle–ductile transitions in carbon nanotubes.
Chemical details
Theactivation energy
In the Arrhenius model of reaction rates, activation energy is the minimum amount of energy that must be available to reactants for a chemical reaction to occur. The activation energy (''E''a) of a reaction is measured in kilojoules per mole (k ...
for the simple atomic motion that gives the bond-rotation apparent in a Stone–Wales defects is fairly high—a barrier of several electronvolt
In physics, an electronvolt (symbol eV), also written electron-volt and electron volt, is the measure of an amount of kinetic energy gained by a single electron accelerating through an Voltage, electric potential difference of one volt in vacuum ...
s. but various processes can create the defects at substantially lower energies than might be expected.
The rearrangement creates a structure with less resonance stabilization
In chemistry, resonance, also called mesomerism, is a way of describing bonding in certain molecules or polyatomic ions by the combination of several contributing structures (or ''forms'', also variously known as ''resonance structures'' or '' ...
among the sp2 atoms involved and higher strain energy
In physics, the elastic potential energy gained by a wire during elongation with a tensile (stretching) or compressive (contractile) force is called strain energy. For linearly elastic materials, strain energy is:
: U = \frac 1 2 V \sigma \v ...
in the local structure. As a result, the defect creates a region with greater chemical reactivity, including acting as a nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
and creating a preferred site for binding to hydrogen atoms. The high affinity of these defects for hydrogen, coupled with the large surface area of the bulk material, might make these defects an important aspect in the use of carbon nanomaterials for hydrogen storage. Incorporation of defects along a carbon-nanotube network can program a carbon-nanotube circuit to enhance the conductance along a specific path. In this scenario, the defects lead to a charge delocalization, which redirects an incoming electron down a given trajectory.
References
External links
* {{DEFAULTSORT:Stone-Wales defect Carbon nanotubes Crystallographic defects