Steroid Sparing Agent on:
[Wikipedia]
[Google]
[Amazon]
A steroid is an
 Steroids are named after the steroid
Steroids are named after the steroid
File:Testosteron.svg, alt=Chemical diagram,
In addition to the ring scissions (cleavages), expansions and contractions (cleavage and reclosing to a larger or smaller rings)—all variations in the carbon-carbon bond framework—steroids can also vary:
* in the
 The hundreds of steroids found in animals, fungi, and
The hundreds of steroids found in animals, fungi, and
 The mevalonate pathway (also called HMG-CoA reductase pathway) begins with acetyl-CoA and ends with dimethylallyl pyrophosphate, dimethylallyl diphosphate (DMAPP) and isopentenyl pyrophosphate, isopentenyl diphosphate (IPP).
DMAPP and IPP donate isoprene units, which are assembled and modified to form terpenes and terpenoid, isoprenoids (a large class of lipids, which include the carotenoids and form the largest class of plant
The mevalonate pathway (also called HMG-CoA reductase pathway) begins with acetyl-CoA and ends with dimethylallyl pyrophosphate, dimethylallyl diphosphate (DMAPP) and isopentenyl pyrophosphate, isopentenyl diphosphate (IPP).
DMAPP and IPP donate isoprene units, which are assembled and modified to form terpenes and terpenoid, isoprenoids (a large class of lipids, which include the carotenoids and form the largest class of plant
 Steroidogenesis is the biological process by which steroids are generated from cholesterol and changed into other steroids. The metabolic pathway, pathways of steroidogenesis differ among species. The major classes of steroid hormones, as noted above (with their prominent members and functions), are the
Steroidogenesis is the biological process by which steroids are generated from cholesterol and changed into other steroids. The metabolic pathway, pathways of steroidogenesis differ among species. The major classes of steroid hormones, as noted above (with their prominent members and functions), are the
organic compound
Some chemical authorities define an organic compound as a chemical compound that contains a carbon–hydrogen or carbon–carbon bond; others consider an organic compound to be any chemical compound that contains carbon. For example, carbon-co ...
with four fused rings (designated A, B, C, and D) arranged in a specific molecular configuration The molecular configuration of a molecule is the ''permanent'' geometry that results from the spatial arrangement of its bonds. The ability of the same set of atoms to form two or more molecules with different configurations is stereoisomerism. Th ...
.
Steroids have two principal biological functions: as important components of cell membrane
The cell membrane (also known as the plasma membrane or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of a cell from the outside environment (the extr ...
s that alter membrane fluidity
In biology, membrane fluidity refers to the viscosity of the lipid bilayer of a cell membrane or a synthetic lipid membrane. Lipid packing can influence the fluidity of the membrane. Viscosity of the membrane can affect the rotation and diffusion ...
; and as signaling molecules
In biology, cell signaling (cell signalling in British English) is the process by which a cell interacts with itself, other cells, and the environment. Cell signaling is a fundamental property of all cellular life in both prokaryotes and eukary ...
. Examples include the lipid
Lipids are a broad group of organic compounds which include fats, waxes, sterols, fat-soluble vitamins (such as vitamins A, D, E and K), monoglycerides, diglycerides, phospholipids, and others. The functions of lipids include storing ...
cholesterol
Cholesterol is the principal sterol of all higher animals, distributed in body Tissue (biology), tissues, especially the brain and spinal cord, and in Animal fat, animal fats and oils.
Cholesterol is biosynthesis, biosynthesized by all anima ...
, sex hormones estradiol
Estradiol (E2), also called oestrogen, oestradiol, is an estrogen steroid hormone and the major female sex hormone. It is involved in the regulation of female reproductive cycles such as estrous and menstrual cycles. Estradiol is responsible ...
and testosterone
Testosterone is the primary male sex hormone and androgen in Male, males. In humans, testosterone plays a key role in the development of Male reproductive system, male reproductive tissues such as testicles and prostate, as well as promoting se ...
, anabolic steroid
Anabolic steroids, also known as anabolic-androgenic steroids (AAS), are a class of drugs that are structurally related to testosterone, the main male sex hormone, and produce effects by binding to the androgen receptor (AR). Anabolism, Anaboli ...
s, and the anti-inflammatory
Anti-inflammatory is the property of a substance or treatment that reduces inflammation, fever or swelling. Anti-inflammatory drugs, also called anti-inflammatories, make up about half of analgesics. These drugs reduce pain by inhibiting mechan ...
corticosteroid drug dexamethasone
Dexamethasone is a fluorinated glucocorticoid medication used to treat rheumatic problems, a number of skin diseases, severe allergies, asthma, chronic obstructive pulmonary disease (COPD), croup, brain swelling, eye pain following eye su ...
. Hundreds of steroids are found in fungi
A fungus (: fungi , , , or ; or funguses) is any member of the group of eukaryotic organisms that includes microorganisms such as yeasts and mold (fungus), molds, as well as the more familiar mushrooms. These organisms are classified as one ...
, plant
Plants are the eukaryotes that form the Kingdom (biology), kingdom Plantae; they are predominantly Photosynthesis, photosynthetic. This means that they obtain their energy from sunlight, using chloroplasts derived from endosymbiosis with c ...
s, and animal
Animals are multicellular, eukaryotic organisms in the Biology, biological Kingdom (biology), kingdom Animalia (). With few exceptions, animals heterotroph, consume organic material, Cellular respiration#Aerobic respiration, breathe oxygen, ...
s. All steroids are manufactured in cells from a sterol
A sterol is any organic compound with a Skeletal formula, skeleton closely related to Cholestanol, cholestan-3-ol. The simplest sterol is gonan-3-ol, which has a formula of , and is derived from that of gonane by replacement of a hydrogen atom on ...
: cholesterolanosterol
Lanosterol is a tetracyclic triterpenoid and is the compound from which all animal and fungal steroids are derived. By contrast, plant steroids are produced via cycloartenol. In the eyes of vertebrates, lanosterol is a natural constituent, havin ...
(opisthokonts
The opisthokonts () are a broad group of eukaryotes, including both the animal and fungus kingdoms. The opisthokonts, previously called the "Fungi/Metazoa group", are generally recognized as a clade. Opisthokonts together with Apusomonadida and ...
), or cycloartenol
Cycloartenol is an important triterpenoid often found in plants. It belongs to the sterol class of steroids. It is the starting point for the synthesis of almost all plant steroids, making them chemically distinct from the steroids of fungi and a ...
(plants). All three of these molecules are produced via cyclization
A cyclic compound (or ring compound) is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where ...
of the triterpene
Triterpenes are a class of terpenes composed of six isoprene units with the molecular formula C30H48; they may also be thought of as consisting of three terpene units. Animals, plants and fungi all produce triterpenes, including squalene, the pre ...
squalene
Squalene is an organic compound. It is a triterpene with the formula C30H50. It is a colourless oil, although impure samples appear yellow. It was originally obtained from shark liver oil (hence its name, as '' Squalus'' is a genus of sharks). ...
.
Structure
The steroid nucleus ( core structure) is calledgonane
Gonane (cyclopentanoperhydrophenanthrene) is a chemical compound with formula , whose structure consists of four hydrocarbon rings fused together: three cyclohexane units and one cyclopentane. It can also be viewed as the result of fusing a cyc ...
(cyclopentanoperhydrophenanthrene). It is typically composed of seventeen carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
atoms, bonded in four fused rings: three six-member cyclohexane
Cyclohexane is a cycloalkane with the molecular formula . Cyclohexane is non-polar. Cyclohexane is a colourless, flammable liquid with a distinctive detergent-like odor, reminiscent of cleaning products (in which it is sometimes used). Cyclohexan ...
rings (rings A, B and C in the first illustration) and one five-member cyclopentane
Cyclopentane (also called C pentane) is a highly flammable alicyclic compound, alicyclic hydrocarbon with chemical formula C5H10, C5H10 and CAS number 287-92-3, consisting of a ring of five carbon atoms each bonded with two hydrogen atoms above and ...
ring (the D ring). Steroids vary by the functional groups
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
attached to this four-ring core and by the oxidation state
In chemistry, the oxidation state, or oxidation number, is the hypothetical Electrical charge, charge of an atom if all of its Chemical bond, bonds to other atoms are fully Ionic bond, ionic. It describes the degree of oxidation (loss of electrons ...
of the rings. Sterol
A sterol is any organic compound with a Skeletal formula, skeleton closely related to Cholestanol, cholestan-3-ol. The simplest sterol is gonan-3-ol, which has a formula of , and is derived from that of gonane by replacement of a hydrogen atom on ...
s are forms of steroids with a hydroxy group
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ...
at position three and a skeleton derived from cholestane
Cholestane is a saturated tetracyclic steroid biosynthetically derived from triterpenoids. This 27-carbon biomarker is produced by diagenesis of cholesterol and is one of the most abundant biomarkers in the rock record. Presence of cholestane, it ...
. ''Also available with the same authors at'' ; ''Also available online at'' Also available in print at Steroids can also be more radically modified, such as by changes to the ring structure, for example, cutting
Cutting is the separation or opening of a physical object, into two or more portions, through the application of an acutely directed force.
Implements commonly used for wikt:cut, cutting are the knife and saw, or in medicine and science the sca ...
one of the rings. Cutting Ring B produces secosteroid
A secosteroid () is a type of steroid with a "broken" ring. The word ''secosteroid ''derives from the Latin verb ''secare'' meaning "to cut", and 'steroid'. Secosteroids are described as a subclass of steroids under the IUPAC nomenclature. Some ...
s one of which is vitamin D3.
Nomenclature
Rings and functional groups
 Steroids are named after the steroid
Steroids are named after the steroid cholesterol
Cholesterol is the principal sterol of all higher animals, distributed in body Tissue (biology), tissues, especially the brain and spinal cord, and in Animal fat, animal fats and oils.
Cholesterol is biosynthesis, biosynthesized by all anima ...
which was first described in gall stones from Ancient Greek
Ancient Greek (, ; ) includes the forms of the Greek language used in ancient Greece and the classical antiquity, ancient world from around 1500 BC to 300 BC. It is often roughly divided into the following periods: Mycenaean Greek (), Greek ...
''chole-'' 'bile
Bile (from Latin ''bilis''), also known as gall, is a yellow-green/misty green fluid produced by the liver of most vertebrates that aids the digestion of lipids in the small intestine. In humans, bile is primarily composed of water, is pro ...
' and ''stereos'' 'solid'.
Gonane
Gonane (cyclopentanoperhydrophenanthrene) is a chemical compound with formula , whose structure consists of four hydrocarbon rings fused together: three cyclohexane units and one cyclopentane. It can also be viewed as the result of fusing a cyc ...
, also known as steran or cyclopentanoperhydrophenanthrene, the nucleus of all steroids and sterols, is composed of seventeen carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
atoms in carbon-carbon bonds forming four fused rings in a three-dimensional shape. The three cyclohexane
Cyclohexane is a cycloalkane with the molecular formula . Cyclohexane is non-polar. Cyclohexane is a colourless, flammable liquid with a distinctive detergent-like odor, reminiscent of cleaning products (in which it is sometimes used). Cyclohexan ...
rings (A, B, and C in the first illustration) form the skeleton of a perhydro derivative of phenanthrene
Phenanthrene is a polycyclic aromatic hydrocarbon (PAH) with formula C14H10, consisting of three fused benzene rings. It is a colorless, crystal-like solid, but can also appear yellow. Phenanthrene is used to make dyes, plastics, pesticides, expl ...
. The D ring has a cyclopentane
Cyclopentane (also called C pentane) is a highly flammable alicyclic compound, alicyclic hydrocarbon with chemical formula C5H10, C5H10 and CAS number 287-92-3, consisting of a ring of five carbon atoms each bonded with two hydrogen atoms above and ...
structure. When the two methyl groups and eight carbon side chain
In organic chemistry and biochemistry, a side chain is a substituent, chemical group that is attached to a core part of the molecule called the "main chain" or backbone chain, backbone. The side chain is a hydrocarbon branching element of a mo ...
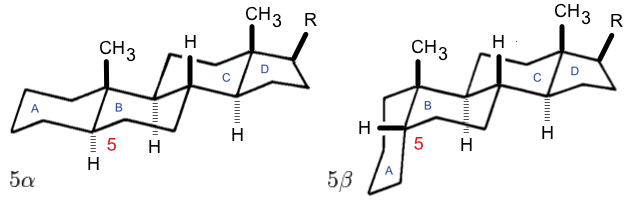
s (at C-17, as shown for cholesterol) are present, the steroid is said to have a cholestane framework. The two common 5α and 5β stereoisomeric forms of steroids exist because of differences in the side of the largely planar ring system where the hydrogen (H) atom at carbon-5 is attached, which results in a change in steroid A-ring conformation. Isomerisation at the C-21 side chain produces a parallel series of compounds, referred to as isosteroids.
Examples of steroid structures are:
Testosterone
Testosterone is the primary male sex hormone and androgen in Male, males. In humans, testosterone plays a key role in the development of Male reproductive system, male reproductive tissues such as testicles and prostate, as well as promoting se ...
, the principal male sex hormone
Sex hormones, also known as sex steroids, gonadocorticoids and gonadal steroids, are steroid hormones that interact with vertebrate steroid hormone receptors. The sex hormones include the androgens, estrogens, and progestogens. Their effects a ...
and an anabolic steroid
Anabolic steroids, also known as anabolic-androgenic steroids (AAS), are a class of drugs that are structurally related to testosterone, the main male sex hormone, and produce effects by binding to the androgen receptor (AR). Anabolism, Anaboli ...
File:Cholsäure.svg, alt=Chemical diagram, Cholic acid
Cholic acid, also known as 3α,7α,12α-trihydroxy-5β-cholan-24-oic acid is a primary bile acid that is insoluble in water (soluble in alcohol and acetic acid), it is a white crystalline substance. Salts of cholic acid are called cholates. Cho ...
, a bile acid
Bile acids are steroid acids found predominantly in the bile of mammals and other vertebrates. Diverse bile acids are synthesized in the liver in peroxisomes. Bile acids are conjugated with taurine or glycine residues to give anions called bile ...
File:Dexamethasone structure.svg, alt=Chemical diagram, Dexamethasone
Dexamethasone is a fluorinated glucocorticoid medication used to treat rheumatic problems, a number of skin diseases, severe allergies, asthma, chronic obstructive pulmonary disease (COPD), croup, brain swelling, eye pain following eye su ...
, a synthetic corticosteroid
Corticosteroids are a class of steroid hormones that are produced in the adrenal cortex of vertebrates, as well as the synthetic analogues of these hormones. Two main classes of corticosteroids, glucocorticoids and mineralocorticoids, are invo ...
drug
File:Lanosterin.svg, alt=Chemical diagram, Lanosterol
Lanosterol is a tetracyclic triterpenoid and is the compound from which all animal and fungal steroids are derived. By contrast, plant steroids are produced via cycloartenol. In the eyes of vertebrates, lanosterol is a natural constituent, havin ...
, the biosynthetic
Biosynthesis, i.e., chemical synthesis occurring in biological contexts, is a term most often referring to multi-step, enzyme- catalyzed processes where chemical substances absorbed as nutrients (or previously converted through biosynthesis) serve ...
precursor to animal steroids. The number of carbons (30) indicates its triterpenoid
Triterpenes are a class of terpenes composed of six isoprene units with the molecular formula C30H48; they may also be thought of as consisting of three terpene units. Animals, plants and fungi all produce triterpenes, including squalene, the pre ...
classification.
File:Progesteron.svg, alt=Chemical diagram, Progesterone
Progesterone (; P4) is an endogenous steroid and progestogen sex hormone involved in the menstrual cycle, pregnancy, and embryogenesis of humans and other species. It belongs to a group of steroid hormones called the progestogens and is the ma ...
, a steroid hormone involved in the female menstrual cycle, pregnancy, and embryogenesis
File:Medrogestone.png, alt=Chemical diagram, Medrogestone
Medrogestone, sold under the brand name Colprone among others, is a progestin medication which has been used in menopausal hormone therapy and in the treatment of gynecological disorders. It is available both alone and in combination with an est ...
, a synthetic drug with effects similar to progesterone
File:Sitosterol structure.svg, alt=Chemical diagram, β-Sitosterol, a plant or phytosterol
Phytosterols are phytosteroids, similar to cholesterol, that serve as structural components of biological membranes of plants. They encompass plant sterols and stanol ester, stanols. More than 250 sterols and related compounds have been identified ...
, with a fully branched hydrocarbon side chain at C-17 and an hydroxyl group at C-3
bond order
In chemistry, bond order is a formal measure of the multiplicity of a covalent bond between two atoms. As introduced by Gerhard Herzberg, building off of work by R. S. Mulliken and Friedrich Hund, bond order is defined as the difference between t ...
s within the rings,
* in the number of methyl groups attached to the ring (and, when present, on the prominent side chain at C17),
* in the functional groups attached to the rings and side chain, and
* in the configuration
Configuration or configurations may refer to:
Computing
* Computer configuration or system configuration
* Configuration file, a software file used to configure the initial settings for a computer program
* Configurator, also known as choice board ...
of groups attached to the rings and chain.
For instance, sterol
A sterol is any organic compound with a Skeletal formula, skeleton closely related to Cholestanol, cholestan-3-ol. The simplest sterol is gonan-3-ol, which has a formula of , and is derived from that of gonane by replacement of a hydrogen atom on ...
s such as cholesterol and lanosterol have a hydroxyl group
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ...
attached at position C-3, while testosterone
Testosterone is the primary male sex hormone and androgen in Male, males. In humans, testosterone plays a key role in the development of Male reproductive system, male reproductive tissues such as testicles and prostate, as well as promoting se ...
and progesterone
Progesterone (; P4) is an endogenous steroid and progestogen sex hormone involved in the menstrual cycle, pregnancy, and embryogenesis of humans and other species. It belongs to a group of steroid hormones called the progestogens and is the ma ...
have a carbonyl (oxo substituent) at C-3. Among these compounds, only lanosterol
Lanosterol is a tetracyclic triterpenoid and is the compound from which all animal and fungal steroids are derived. By contrast, plant steroids are produced via cycloartenol. In the eyes of vertebrates, lanosterol is a natural constituent, havin ...
has two methyl groups at C-4. Cholesterol which has a C-5 to C-6 double bond, differs from testosterone and progesterone which have a C-4 to C-5 double bond.
Naming convention
Almost all biologically relevant steroids can be presented as a derivative of a parentcholesterol
Cholesterol is the principal sterol of all higher animals, distributed in body Tissue (biology), tissues, especially the brain and spinal cord, and in Animal fat, animal fats and oils.
Cholesterol is biosynthesis, biosynthesized by all anima ...
-like hydrocarbon
In organic chemistry, a hydrocarbon is an organic compound consisting entirely of hydrogen and carbon. Hydrocarbons are examples of group 14 hydrides. Hydrocarbons are generally colourless and Hydrophobe, hydrophobic; their odor is usually fain ...
structure that serves as a skeleton
A skeleton is the structural frame that supports the body of most animals. There are several types of skeletons, including the exoskeleton, which is a rigid outer shell that holds up an organism's shape; the endoskeleton, a rigid internal fra ...
. These parent structures have specific names, such as pregnane
Pregnane, also known as 17β-ethylandrostane or as 10β,13β-dimethyl-17β-ethylgonane, is a C21 steroid and, indirectly, a parent of progesterone. It is a parent hydrocarbon for two series of steroids stemming from 5α-pregnane (originally all ...
, androstane
Androstane is a C19 steroidal hydrocarbon with a gonane core. Androstane can exist as either of two isomers, known as 5α-androstane and 5β-androstane.
File:androstane.png, 5α-Androstane
File:androstane 5beta.png, 5β-Androstane
Pharmacolog ...
, etc. The derivatives carry various functional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
s called suffixes or prefixes after the respective numbers, indicating their position in the steroid nucleus. There are widely used trivial steroid names of natural origin with significant biologic activity, such as progesterone
Progesterone (; P4) is an endogenous steroid and progestogen sex hormone involved in the menstrual cycle, pregnancy, and embryogenesis of humans and other species. It belongs to a group of steroid hormones called the progestogens and is the ma ...
, testosterone
Testosterone is the primary male sex hormone and androgen in Male, males. In humans, testosterone plays a key role in the development of Male reproductive system, male reproductive tissues such as testicles and prostate, as well as promoting se ...
or cortisol
Cortisol is a steroid hormone in the glucocorticoid class of hormones and a stress hormone. When used as medication, it is known as hydrocortisone.
Cortisol is produced in many animals, mainly by the ''zona fasciculata'' of the adrenal corte ...
. Some of these names are defined in The Nomenclature of Steroids. These trivial names can also be used as a base to derive new names, however, by adding prefixes only rather than suffixes, e.g., the steroid 17α-hydroxyprogesterone has a hydroxy group
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ...
(-OH) at position 17 of the steroid nucleus comparing to progesterone.
The letters α and β denote absolute stereochemistry
Stereochemistry, a subdiscipline of chemistry, studies the spatial arrangement of atoms that form the structure of molecules and their manipulation. The study of stereochemistry focuses on the relationships between stereoisomers, which are defined ...
at chiral centers
In stereochemistry, a stereocenter of a molecule is an atom (center), axis or plane that is the focus of stereoisomerism; that is, when having at least three different groups bound to the stereocenter, interchanging any two different groups cr ...
—a specific nomenclature distinct from the R/S convention of organic chemistry to denote absolute configuration of functional groups, known as Cahn–Ingold–Prelog priority rules
In organic chemistry, the Cahn–Ingold–Prelog (CIP) sequence rules (also the CIP priority convention; named after Robert Sidney Cahn, Christopher Kelk Ingold, and Vladimir Prelog) are a standard process to completely and unequivocally nam ...
. The R/S convention assigns priorities to substituents on a chiral center based on their atomic number. The highest priority group is assigned to the atom with the highest atomic number, and the lowest priority group is assigned to the atom with the lowest atomic number. The molecule is then oriented so that the lowest priority group points away from the viewer, and the remaining three groups are arranged in order of decreasing priority around the chiral center. If this arrangement is clockwise, it is assigned an R configuration; if it is counterclockwise, it is assigned an S configuration. In contrast, steroid nomenclature uses α and β to denote stereochemistry at chiral centers. The α and β designations are based on the orientation of substituents relative to each other in a specific ring system. In general, α refers to a substituent that is oriented towards the plane of the ring system, while β refers to a substituent that is oriented away from the plane of the ring system. In steroids drawn from the standard perspective used in this paper, α-bonds are depicted on figures as dashed wedges and β-bonds as solid wedges.
The name " 11-deoxycortisol" is an example of a derived name that uses cortisol as a parent structure without an oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
atom
Atoms are the basic particles of the chemical elements. An atom consists of a atomic nucleus, nucleus of protons and generally neutrons, surrounded by an electromagnetically bound swarm of electrons. The chemical elements are distinguished fr ...
(hence "deoxy") attached to position 11 (as a part of a hydroxy group). The numbering of positions of carbon
Carbon () is a chemical element; it has chemical symbol, symbol C and atomic number 6. It is nonmetallic and tetravalence, tetravalent—meaning that its atoms are able to form up to four covalent bonds due to its valence shell exhibiting 4 ...
atoms in the steroid nucleus is set in a template found in the Nomenclature of Steroids that is used regardless of whether an atom is present in the steroid in question.
Unsaturated carbons (generally, ones that are part of a double bond) in the steroid nucleus are indicated by changing -ane to -ene. This change was traditionally done in the parent name, adding a prefix to denote the position, with or without Δ (Greek capital delta) which designates unsaturation, for example, 4-pregnene-11β,17α-diol-3,20-dione (also Δ4-pregnene-11β,17α-diol-3,20-dione) or 4-androstene-3,11,17-trione (also Δ4-androstene-3,11,17-trione). However, the Nomenclature of Steroids recommends the locant
In the nomenclature of organic chemistry, a locant is a term to indicate the position of a functional group or substituent within a molecule.
Numeric locants
The International Union of Pure and Applied Chemistry (IUPAC) recommends the use of n ...
of a double bond to be always adjacent to the syllable designating the unsaturation, therefore, having it as a suffix rather than a prefix, and without the use of the Δ character, i.e. pregn-4-ene-11β,17α-diol-3,20-dione or androst-4-ene-3,11,17-trione. The double bond is designated by the lower-numbered carbon atom, i.e. "Δ4-" or "4-ene" means the double bond between positions 4 and 5. The saturation of carbons of a parent steroid can be done by adding "dihydro-" prefix, i.e., a saturation of carbons 4 and 5 of testosterone with two hydrogen
Hydrogen is a chemical element; it has chemical symbol, symbol H and atomic number 1. It is the lightest and abundance of the chemical elements, most abundant chemical element in the universe, constituting about 75% of all baryon, normal matter ...
atoms is 4,5α-dihydrotestosterone or 4,5β-dihydrotestosterone. Generally, when there is no ambiguity, one number of a hydrogen position from a steroid with a saturated bond may be omitted, leaving only the position of the second hydrogen atom, e.g., 5α-dihydrotestosterone
Dihydrotestosterone (DHT, 5α-dihydrotestosterone, 5α-DHT, androstanolone or stanolone) is an endogenous androgen sex steroid and hormone primarily involved in the growth and repair of the prostate and the penis, as well as the production of ...
or 5β-dihydrotestosterone
5β-Dihydrotestosterone (5β-DHT), also known as 5β-androstan-17β-ol-3-one or as etiocholan-17β-ol-3-one, is an etiocholane (5β- androstane) steroid as well as an inactive metabolite of testosterone formed by 5β-reductase in the liver and ...
. The Δ5-steroids are those with a double bond between carbons 5 and 6 and the Δ4 steroids are those with a double bond between carbons 4 and 5.
The abbreviations like " P4" for progesterone
Progesterone (; P4) is an endogenous steroid and progestogen sex hormone involved in the menstrual cycle, pregnancy, and embryogenesis of humans and other species. It belongs to a group of steroid hormones called the progestogens and is the ma ...
and " A4" for androstenedione
Androstenedione, or 4-androstenedione (abbreviated as A4 or Δ4-dione), also known as androst-4-ene-3,17-dione, is an endogenous weak androgen steroid hormone and intermediate in the biosynthesis of estrone and of testosterone from dehydroe ...
for refer to Δ4-steroids, while " P5" for pregnenolone
Pregnenolone (P5), or pregn-5-en-3β-ol-20-one, is an endogenous steroid and precursor/metabolic intermediate in the biosynthesis of most of the steroid hormones, including the progestogens, androgens, estrogens, glucocorticoids, and mineraloc ...
and " A5" for androstenediol
Androstenediol, or 5-androstenediol (abbreviated as A5 or Δ5-diol), also known as androst-5-ene-3β,17β-diol, is an endogenous weak androgen and estrogen steroid hormone and intermediate in the biosynthesis of testosterone from dehydroepiand ...
refer to Δ5-steroids.
The suffix -ol denotes a hydroxy group
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydroxy ...
, while the suffix -one denotes an oxo group. When two or three identical groups are attached to the base structure at different positions, the suffix is indicated as -diol or -triol for hydroxy, and -dione or -trione for oxo groups, respectively. For example, 5α-pregnane-3α,17α-diol-20-one has a hydrogen atom at the 5α position (hence the "5α-" prefix), two hydroxy groups (-OH) at the 3α and 17α positions (hence "3α,17α-diol" suffix) and an oxo group (=O) at the position 20 (hence the "20-one" suffix). However, erroneous use of suffixes can be found, e.g., "5α-pregnan-17α-diol-3,11,20-trione" 'sic''— since it has just one hydroxy group (at 17α) rather than two, then the suffix should be -ol, rather than -diol, so that the correct name to be "5α-pregnan-17α-ol-3,11,20-trione".
According to the rule set in the Nomenclature of Steroids, the terminal "e" in the parent structure name should be elided before the vowel
A vowel is a speech sound pronounced without any stricture in the vocal tract, forming the nucleus of a syllable. Vowels are one of the two principal classes of speech sounds, the other being the consonant. Vowels vary in quality, in loudness a ...
(the presence or absence of a number does not affect such elision). This means, for instance, that if the suffix immediately appended to the parent structure name begins with a vowel, the trailing "e" is removed from that name. An example of such removal is " 5α-pregnan-17α-ol-3,20-dione", where the last "e" of "pregnane
Pregnane, also known as 17β-ethylandrostane or as 10β,13β-dimethyl-17β-ethylgonane, is a C21 steroid and, indirectly, a parent of progesterone. It is a parent hydrocarbon for two series of steroids stemming from 5α-pregnane (originally all ...
" is dropped due to the vowel ("o") at the beginning of the suffix -ol. Some authors incorrectly use this rule, eliding the terminal "e" where it should be kept, or vice versa.
The term "11-oxygenated" refers to the presence of an oxygen atom as an oxo (=O) or hydroxy (-OH) substituent at carbon 11. "Oxygenated" is consistently used within the chemistry of the steroids since the 1950s. Some studies use the term "11-oxyandrogens" as an abbreviation for 11-oxygenated androgens, to emphasize that they all have an oxygen atom attached to carbon at position 11. However, in chemical nomenclature, the prefix "oxy" is associated with ether functional groups, i.e., a compound
Compound may refer to:
Architecture and built environments
* Compound (enclosure), a cluster of buildings having a shared purpose, usually inside a fence or wall
** Compound (fortification), a version of the above fortified with defensive struc ...
with an oxygen atom connected to two alkyl
In organic chemistry, an alkyl group is an alkane missing one hydrogen.
The term ''alkyl'' is intentionally unspecific to include many possible substitutions.
An acyclic alkyl has the general formula of . A cycloalkyl group is derived from a cy ...
or aryl
In organic chemistry, an aryl is any functional group or substituent derived from an aromatic ring, usually an aromatic hydrocarbon, such as phenyl and naphthyl. "Aryl" is used for the sake of abbreviation or generalization, and "Ar" is used ...
groups (R-O-R), therefore, using "oxy" within the name of a steroid class may be misleading. One can find clear examples of "oxygenated" to refer to a broad class of organic molecules containing a variety of oxygen containing functional groups in other domains of organic chemistry, and it is appropriate to use this convention.
Even though "keto" is a standard prefix in organic chemistry, the 1989 recommendations of the Joint Commission on Biochemical Nomenclature discourage the application of the prefix "keto" for steroid names, and favor the prefix "oxo" (e.g., 11-oxo steroids rather than 11-keto steroids), because "keto" includes the carbon that is part of the steroid nucleus and the same carbon atom should not be specified twice.
Species distribution
Steroids are present across all domains of life, includingbacteria
Bacteria (; : bacterium) are ubiquitous, mostly free-living organisms often consisting of one Cell (biology), biological cell. They constitute a large domain (biology), domain of Prokaryote, prokaryotic microorganisms. Typically a few micr ...
, archaea
Archaea ( ) is a Domain (biology), domain of organisms. Traditionally, Archaea only included its Prokaryote, prokaryotic members, but this has since been found to be paraphyletic, as eukaryotes are known to have evolved from archaea. Even thou ...
, and eukaryote
The eukaryotes ( ) constitute the Domain (biology), domain of Eukaryota or Eukarya, organisms whose Cell (biology), cells have a membrane-bound cell nucleus, nucleus. All animals, plants, Fungus, fungi, seaweeds, and many unicellular organisms ...
s. In eukaryotes, steroids are particularly abundant in fungi, plants, and animals.
Eukaryotic
Eukaryotic
The eukaryotes ( ) constitute the Domain (biology), domain of Eukaryota or Eukarya, organisms whose Cell (biology), cells have a membrane-bound cell nucleus, nucleus. All animals, plants, Fungus, fungi, seaweeds, and many unicellular organisms ...
cells, encompassing animals, plants, fungi, and protists, are characterized by their complex cellular structures, including a true nucleus and membrane-bound organelles. Sterols, a subgroup of steroids, play crucial roles in maintaining membrane fluidity, supporting cell signaling, and enhancing stress tolerance. These compounds are integral to eukaryotic membranes, where they contribute to membrane integrity and functionality.
During eukaryogenesis
Eukaryogenesis, the process which created the eukaryotic cell and lineage, is a milestone in the evolution of life, since eukaryotes include all complex cells and almost all multicellular organisms. The process is widely agreed to have involved ...
—the evolutionary process that gave rise to modern eukaryotic cells—steroids likely facilitated the endosymbiotic acquisition of mitochondria.
Prokaryotic
Although sterol biosynthesis is rare in prokaryotes, certain bacteria, including ''Methylococcus capsulatus
''Methylococcus capsulatus'' is an obligately methanotrophic gram-negative, non-motile coccoid bacterium. ''M. capsulatus'' are thermotolerant; their cells are encapsulated and tend to have a diplococcoid shape. The cell wall is composed o ...
'', specific methanotroph
Methanotrophs (sometimes called methanophiles) are prokaryotes that metabolize methane as their source of carbon and chemical energy. They are bacteria or archaea, can grow aerobically or anaerobically, and require single-carbon compounds to ...
s, myxobacteria
The myxobacteria ("slime bacteria") are a group of bacteria that predominantly live in the soil and feed on insoluble organic substances. The myxobacteria have very large genomes relative to other bacteria, e.g. 9–10 million nucleotides except ...
, and the planctomycete
The Planctomycetota are a phylum of widely distributed bacteria, occurring in both aquatic and terrestrial habitats. They play a considerable role in global carbon and nitrogen cycles, with many species of this phylum capable of anaerobic ammoniu ...
''Gemmata obscuriglobus
''Gemmata obscuriglobus'' is a species of Gram-negative, aerobic, heterotrophic bacteria of the phylum Planctomycetota. ''G. obscuriglobus'' occur in freshwater habitats and was first described in 1984, and is the only described species in its ge ...
'', are capable of producing sterols. In ''G. obscuriglobus'', sterols are essential for cell viability, but their roles in other bacteria remain poorly understood.
Prokaryotic sterol synthesis involves the tetracyclic steroid framework, as found in myxobacteria
The myxobacteria ("slime bacteria") are a group of bacteria that predominantly live in the soil and feed on insoluble organic substances. The myxobacteria have very large genomes relative to other bacteria, e.g. 9–10 million nucleotides except ...
, as well as hopanoids
Hopanoids are a diverse subclass of Triterpene, triterpenoids with the same hydrocarbon skeleton as the compound hopane. This group of Pentacyclic compound, pentacyclic molecules therefore refers to simple hopenes, hopanols and hopanes, but also to ...
, pentacyclic lipids that regulate bacterial membrane functions. These sterol biosynthetic pathways may have originated in bacteria or been transferred from eukaryote
The eukaryotes ( ) constitute the Domain (biology), domain of Eukaryota or Eukarya, organisms whose Cell (biology), cells have a membrane-bound cell nucleus, nucleus. All animals, plants, Fungus, fungi, seaweeds, and many unicellular organisms ...
s.
Sterol synthesis depends on two key enzymes: squalene monooxygenase
Squalene monooxygenase (also called squalene epoxidase) is a eukaryotic enzyme that uses NADPH and diatomic oxygen to oxidize squalene to 2,3-oxidosqualene (squalene epoxide). Squalene epoxidase catalyzes the first oxygenation step in sterol b ...
and oxidosqualene cyclase Oxidosqualene cyclases (OSC) are enzymes involved in cyclization reactions of 2,3-oxidosqualene to form sterols or triterpenes.
There are two major groups of sterol-producing OSC enzymes:
* Cycloartenol synthase (CAS), found in all plants, which ...
. Phylogenetic analyses of oxidosqualene cyclase (Osc) suggest that some bacterial Osc genes may have been acquired via horizontal gene transfer
Horizontal gene transfer (HGT) or lateral gene transfer (LGT) is the movement of genetic material between organisms other than by the ("vertical") transmission of DNA from parent to offspring (reproduction). HGT is an important factor in the e ...
from eukaryotes, as certain bacterial Osc proteins closely resemble their eukaryotic homologs.
Fungal
Fungal steroids include theergosterol
Ergosterol (ergosta-5,7,22-trien-3β-ol) is a mycosterol found in cell membranes of fungi and protozoa, serving many of the same functions that cholesterol serves in animal cells. Because many fungi and protozoa cannot survive without ergostero ...
s, which are involved in maintaining the integrity of the fungal cellular membrane. Various antifungal drugs, such as amphotericin B
Amphotericin B is an antifungal medication used for serious fungal infections and leishmaniasis. The fungal infections it is used to treat include mucormycosis, aspergillosis, blastomycosis, candidiasis, coccidioidomycosis, and cryptococ ...
and azole antifungals
Azoles are a class of five-membered heterocyclic compounds containing a nitrogen atom and at least one other non-carbon atom (i.e. nitrogen, sulfur, or oxygen) as part of the ring.
Their names originate from the Hantzsch–Widman nomenclature. T ...
, utilize this information to kill pathogenic
In biology, a pathogen (, "suffering", "passion" and , "producer of"), in the oldest and broadest sense, is any organism or agent that can produce disease. A pathogen may also be referred to as an infectious agent, or simply a germ.
The term ...
fungi. Fungi can alter their ergosterol content (e.g. through loss of function mutations in the enzymes ERG3 or ERG6, inducing depletion of ergosterol, or mutations that decrease the ergosterol content) to develop resistance to drugs that target ergosterol.
Ergosterol is analogous to the cholesterol
Cholesterol is the principal sterol of all higher animals, distributed in body Tissue (biology), tissues, especially the brain and spinal cord, and in Animal fat, animal fats and oils.
Cholesterol is biosynthesis, biosynthesized by all anima ...
found in the cellular membranes of animals (including humans), or the phytosterols
Phytosterols are phytosteroids, similar to cholesterol, that serve as structural components of biological membranes of plants. They encompass plant sterols and stanols. More than 250 sterols and related compounds have been identified. Free phyto ...
found in the cellular membranes of plants. All mushrooms contain large quantities of ergosterol, in the range of tens to hundreds of milligrams per 100 grams of dry weight. Oxygen is necessary for the synthesis of ergosterol
Ergosterol (ergosta-5,7,22-trien-3β-ol) is a mycosterol found in cell membranes of fungi and protozoa, serving many of the same functions that cholesterol serves in animal cells. Because many fungi and protozoa cannot survive without ergostero ...
in fungi.
Ergosterol is responsible for the vitamin D
Vitamin D is a group of structurally related, fat-soluble compounds responsible for increasing intestinal absorption of calcium, magnesium, and phosphate, along with numerous other biological functions. In humans, the most important compo ...
content found in mushrooms; ergosterol is chemically converted into provitamin D2 by exposure to ultraviolet light
Ultraviolet radiation, also known as simply UV, is electromagnetic radiation of wavelengths of 10–400 nanometers, shorter than that of visible light, but longer than X-rays. UV radiation is present in sunlight and constitutes about 10% of th ...
. Provitamin D2 spontaneously forms vitamin D2. However, not all fungi utilize ergosterol in their cellular membranes; for example, the pathogenic fungal species ''Pneumocystis jirovecii
''Pneumocystis jirovecii'' (previously ''P. carinii'') is a yeast-like fungus of the genus ''Pneumocystis''. The causative organism of ''Pneumocystis'' pneumonia, it is an important human pathogen, particularly among immunocompromised hosts. P ...
'' does not, which has important clinical implications (given the mechanism of action of many antifungal drugs). Using the fungus ''Saccharomyces cerevisiae
''Saccharomyces cerevisiae'' () (brewer's yeast or baker's yeast) is a species of yeast (single-celled fungal microorganisms). The species has been instrumental in winemaking, baking, and brewing since ancient times. It is believed to have be ...
'' as an example, other major steroids include ergosta‐5,7,22,24(28)‐tetraen‐3β‐ol, zymosterol
Zymosterol is an intermediate in cholesterol biosynthesis. Disregarding some intermediate compounds (e.g. 4-4-dimethylzymosterol), lanosterol can be considered a precursor of zymosterol in the cholesterol synthesis pathway. The conversion of zymos ...
, and lanosterol
Lanosterol is a tetracyclic triterpenoid and is the compound from which all animal and fungal steroids are derived. By contrast, plant steroids are produced via cycloartenol. In the eyes of vertebrates, lanosterol is a natural constituent, havin ...
. ''S. cerevisiae'' utilizes 5,6‐dihydroergosterol in place of ergosterol in its cell membrane.
Plant
Plant steroids include steroidalalkaloid
Alkaloids are a broad class of natural product, naturally occurring organic compounds that contain at least one nitrogen atom. Some synthetic compounds of similar structure may also be termed alkaloids.
Alkaloids are produced by a large varie ...
s found in Solanaceae
Solanaceae (), commonly known as the nightshades, is a family of flowering plants in the order Solanales. It contains approximately 2,700 species, several of which are used as agricultural crops, medicinal plants, and ornamental plants. Many me ...
and Melanthiaceae
Melanthiaceae, also called the bunchflower family, is a family (biology), family of flowering plant, flowering herbaceous perennial plants native to the Northern Hemisphere. Along with many other lilioid monocots, early authors considered member ...
(specially the genus Veratrum
''Veratrum'' is a genus of flowering plants in the family Melanthiaceae. It occurs in damp habitats across much of temperate and subarctic Europe, Asia, and North America.
''Veratrum'' species are vigorous herbaceous perennials with highly poiso ...
), cardiac glycoside
Cardiac glycosides are a class of organic compounds that increase the output force of the heart and decrease its rate of contractions by inhibiting the cellular sodium-potassium ATPase pump. Their beneficial medical uses include treatments for ...
s, the phytosterol
Phytosterols are phytosteroids, similar to cholesterol, that serve as structural components of biological membranes of plants. They encompass plant sterols and stanol ester, stanols. More than 250 sterols and related compounds have been identified ...
s and the brassinosteroid
Brassinosteroids (BRs or less commonly BS) are a class of polyhydroxysteroids that have been recognized as a sixth class of
plant hormones and may have utility as anticancer drugs for treating endocrine-responsive cancers by inducing apoptosis of ...
s (which include several plant hormones).
Animal
Animal steroids include compounds ofvertebrate
Vertebrates () are animals with a vertebral column (backbone or spine), and a cranium, or skull. The vertebral column surrounds and protects the spinal cord, while the cranium protects the brain.
The vertebrates make up the subphylum Vertebra ...
and insect
Insects (from Latin ') are Hexapoda, hexapod invertebrates of the class (biology), class Insecta. They are the largest group within the arthropod phylum. Insects have a chitinous exoskeleton, a three-part body (Insect morphology#Head, head, ...
origin, the latter including ecdysteroid
Ecdysteroids are arthropod steroid hormones that are mainly responsible for molting (ecdysis), development and, to a lesser extent, reproduction; examples of ecdysteroids include ecdysone, 20-hydroxyecdysone (ecdysterone), turkesterone and 2- ...
s such as ecdysterone (controlling molting in some species). Vertebrate examples include the steroid hormones
A steroid hormone is a steroid that acts as a hormone. Steroid hormones can be grouped into two classes: corticosteroids (typically made in the adrenal cortex, hence ''cortico-'') and sex steroids (typically made in the gonads or placenta). Withi ...
and cholesterol; the latter is a structural component of cell membranes
The cell membrane (also known as the plasma membrane or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of a cell from the outside environment (the extra ...
that helps determine the fluidity of cell membranes
The cell membrane (also known as the plasma membrane or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of a cell from the outside environment (the extra ...
and is a principal constituent of plaque (implicated in atherosclerosis
Atherosclerosis is a pattern of the disease arteriosclerosis, characterized by development of abnormalities called lesions in walls of arteries. This is a chronic inflammatory disease involving many different cell types and is driven by eleva ...
). Steroid hormones include:
* Sex hormone
Sex hormones, also known as sex steroids, gonadocorticoids and gonadal steroids, are steroid hormones that interact with vertebrate steroid hormone receptors. The sex hormones include the androgens, estrogens, and progestogens. Their effects a ...
s, which influence sex differences
Sexual dimorphism is the condition where sexes of the same species exhibit different morphological characteristics, including characteristics not directly involved in reproduction. The condition occurs in most dioecious species, which consi ...
and support puberty
Puberty is the process of physical changes through which a child's body matures into an adult body capable of sexual reproduction. It is initiated by hormonal signals from the brain to the gonads: the ovaries in a female, the testicles i ...
and reproduction
Reproduction (or procreation or breeding) is the biological process by which new individual organisms – "offspring" – are produced from their "parent" or parents. There are two forms of reproduction: Asexual reproduction, asexual and Sexual ...
. These include androgen
An androgen (from Greek ''andr-'', the stem of the word meaning ) is any natural or synthetic steroid hormone that regulates the development and maintenance of male characteristics in vertebrates by binding to androgen receptors. This includes ...
s, estrogen
Estrogen (also spelled oestrogen in British English; see spelling differences) is a category of sex hormone responsible for the development and regulation of the female reproductive system and secondary sex characteristics. There are three ...
s, and progestogen
Progestogens, also sometimes written progestins, progestagens or gestagens, are a class of natural or synthetic steroid hormones that bind to and activate the progesterone receptors (PR). Progesterone is the major and most important progestoge ...
s.
* Corticosteroid
Corticosteroids are a class of steroid hormones that are produced in the adrenal cortex of vertebrates, as well as the synthetic analogues of these hormones. Two main classes of corticosteroids, glucocorticoids and mineralocorticoids, are invo ...
s, including most synthetic steroid drugs, with natural product
A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical s ...
classes the glucocorticoid
Glucocorticoids (or, less commonly, glucocorticosteroids) are a class of corticosteroids, which are a class of steroid hormones. Glucocorticoids are corticosteroids that bind to the glucocorticoid receptor that is present in almost every vertebra ...
s (which regulate many aspects of metabolism
Metabolism (, from ''metabolē'', "change") is the set of life-sustaining chemical reactions in organisms. The three main functions of metabolism are: the conversion of the energy in food to energy available to run cellular processes; the co ...
and immune function
The immune system is a network of biological systems that protects an organism from diseases. It detects and responds to a wide variety of pathogens, from viruses to bacteria, as well as cancer cells, parasitic worms, and also objects such as ...
) and the mineralocorticoid
Mineralocorticoids are a class of corticosteroids, which in turn are a class of steroid hormones. Mineralocorticoids are produced in the adrenal cortex and influence salt and water balances (electrolyte balance and fluid balance). The primary ...
s (which help maintain blood volume and control renal
In humans, the kidneys are two reddish-brown bean-shaped blood-filtering organs that are a multilobar, multipapillary form of mammalian kidneys, usually without signs of external lobulation. They are located on the left and right in the retrop ...
excretion of electrolyte
An electrolyte is a substance that conducts electricity through the movement of ions, but not through the movement of electrons. This includes most soluble Salt (chemistry), salts, acids, and Base (chemistry), bases, dissolved in a polar solven ...
s)
* Anabolic steroid
Anabolic steroids, also known as anabolic-androgenic steroids (AAS), are a class of drugs that are structurally related to testosterone, the main male sex hormone, and produce effects by binding to the androgen receptor (AR). Anabolism, Anaboli ...
s, natural
Nature is an inherent character or constitution, particularly of the ecosphere or the universe as a whole. In this general sense nature refers to the laws, elements and phenomena of the physical world, including life. Although humans are part ...
and synthetic, which interact with androgen receptors to increase muscle and bone synthesis. In popular use, the term "steroids" often refers to anabolic steroids.
Types
By function
The major classes ofsteroid hormones
A steroid hormone is a steroid that acts as a hormone. Steroid hormones can be grouped into two classes: corticosteroids (typically made in the adrenal cortex, hence ''cortico-'') and sex steroids (typically made in the gonads or placenta). Withi ...
, with prominent members and examples of related functions, are:
* Corticosteroid
Corticosteroids are a class of steroid hormones that are produced in the adrenal cortex of vertebrates, as well as the synthetic analogues of these hormones. Two main classes of corticosteroids, glucocorticoids and mineralocorticoids, are invo ...
s:
** Glucocorticoids:
*** Cortisol, a glucocorticoid
Glucocorticoids (or, less commonly, glucocorticosteroids) are a class of corticosteroids, which are a class of steroid hormones. Glucocorticoids are corticosteroids that bind to the glucocorticoid receptor that is present in almost every vertebra ...
whose functions include stress response and immunosuppression
** Mineralocorticoids:
*** Aldosterone, a mineralocorticoid
Mineralocorticoids are a class of corticosteroids, which in turn are a class of steroid hormones. Mineralocorticoids are produced in the adrenal cortex and influence salt and water balances (electrolyte balance and fluid balance). The primary ...
that helps regulate blood pressure through water and electrolyte balance in the Kidney, kidneys
* Sex steroids:
** Progestogens:
*** Progesterone
Progesterone (; P4) is an endogenous steroid and progestogen sex hormone involved in the menstrual cycle, pregnancy, and embryogenesis of humans and other species. It belongs to a group of steroid hormones called the progestogens and is the ma ...
, which regulates cyclical changes in the endometrium of the uterus and maintains a pregnancy
** Androgens:
*** Testosterone
Testosterone is the primary male sex hormone and androgen in Male, males. In humans, testosterone plays a key role in the development of Male reproductive system, male reproductive tissues such as testicles and prostate, as well as promoting se ...
, which contributes to the development and maintenance of male secondary sex characteristics
** Estrogens:
*** Estradiol, which contributes to the development and maintenance of female secondary sex characteristics
Additional classes of steroids include:
* Neurosteroids such as and allopregnanolone
* Bile acids such as taurocholic acid
* Aminosteroid neuromuscular blocking agents (mainly synthetic) such as pancuronium bromide
* Steroidal antiandrogens (mainly synthetic) such as cyproterone acetate
* Steroidogenesis inhibitors (mainly exogenous) such as alfatradiol
* Membrane sterols such as cholesterol
Cholesterol is the principal sterol of all higher animals, distributed in body Tissue (biology), tissues, especially the brain and spinal cord, and in Animal fat, animal fats and oils.
Cholesterol is biosynthesis, biosynthesized by all anima ...
, ergosterol
Ergosterol (ergosta-5,7,22-trien-3β-ol) is a mycosterol found in cell membranes of fungi and protozoa, serving many of the same functions that cholesterol serves in animal cells. Because many fungi and protozoa cannot survive without ergostero ...
, and various phytosterol
Phytosterols are phytosteroids, similar to cholesterol, that serve as structural components of biological membranes of plants. They encompass plant sterols and stanol ester, stanols. More than 250 sterols and related compounds have been identified ...
s
* Toxins such as steroidal saponins and cardenolides/cardiac glycoside
Cardiac glycosides are a class of organic compounds that increase the output force of the heart and decrease its rate of contractions by inhibiting the cellular sodium-potassium ATPase pump. Their beneficial medical uses include treatments for ...
s
As well as the following class of secosteroid
A secosteroid () is a type of steroid with a "broken" ring. The word ''secosteroid ''derives from the Latin verb ''secare'' meaning "to cut", and 'steroid'. Secosteroids are described as a subclass of steroids under the IUPAC nomenclature. Some ...
s (open-ring steroids):
* Vitamin D forms such as ergocalciferol, cholecalciferol, and calcitriol
By structure
Intact ring system
Steroids can be classified based on their chemical composition. One example of how Medical Subject Headings, MeSH performs this classification is available at the Wikipedia MeSH catalog. Examples of this classification include: In biology, it is common to name the above steroid classes by the number of carbon atoms present when referring to hormones: C18-steroids for the estranes (mostly estrogens), C19-steroids for the androstanes (mostly androgens), and C21-steroids for the pregnanes (mostly corticosteroids). The classification "17-ketosteroid" is also important in medicine. The gonane (steroid nucleus) is the parent 17-carbon tetracyclic hydrocarbon molecule with no alkyl sidechains.Cleaved, contracted, and expanded rings
Secosteroids (Latin ''seco'', "to cut") are a subclass of steroidal compounds resulting, Biosynthesis, biosynthetically or conceptually, from scission (cleavage) of parent steroid rings (generally one of the four). Major secosteroid subclasses are defined by the steroid carbon atoms where this scission has taken place. For instance, the prototypical secosteroid cholecalciferol, vitamin D3 (shown), is in the 9,10-secosteroid subclass and derives from the cleavage of carbon atoms C-9 and C-10 of the steroid B-ring; 5,6-secosteroids and 13,14-steroids are similar. Norsteroids (nor-, L. ''norma''; "normal" in chemistry, indicating carbon removal) and homosteroids (homo-, Greek ''homos''; "same", indicating carbon addition) are structural subclasses of steroids formed from biosynthetic steps. The former involves enzymic ring expansion, ring expansion-contraction reactions, and the latter is accomplished (biomimetic synthesis, biomimetically) or (more frequently) through ring closures of open-chain compound, acyclic precursors with more (or fewer) ring atoms than the parent steroid framework. Combinations of these ring alterations are known in nature. For instance, Sheep, ewes who graze on Veratrum, corn lily ingest cyclopamine (shown) and veratramine, two of a sub-family of steroids where the C- and D-rings are contracted and expanded respectively via a biosynthesis, biosynthetic migration of the original C-13 atom. Ingestion of these C-nor-D-homosteroids results in birth defects in lambs: cyclopia from cyclopamine and leg deformity from veratramine. A further C-nor-D-homosteroid (nakiterpiosin) is excreted by Okinawa Prefecture, Okinawan cyanobacteria, cyanobacteriosponges. e.g., ''Terpios hoshinota'', leading to coral mortality from black coral disease. Nakiterpiosin-type steroids are active against the signaling pathway involving the smoothened and hedgehog (cell signaling), hedgehog proteins, a pathway which is hyperactive in a number of cancers.Biological significance
Steroids and their metabolites often function as signal transduction, signalling molecules (the most notable examples are steroid hormones), and steroids and phospholipids are components ofcell membrane
The cell membrane (also known as the plasma membrane or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of a cell from the outside environment (the extr ...
s. Steroids such as cholesterol decrease membrane fluidity
In biology, membrane fluidity refers to the viscosity of the lipid bilayer of a cell membrane or a synthetic lipid membrane. Lipid packing can influence the fluidity of the membrane. Viscosity of the membrane can affect the rotation and diffusion ...
.
Similar to lipid
Lipids are a broad group of organic compounds which include fats, waxes, sterols, fat-soluble vitamins (such as vitamins A, D, E and K), monoglycerides, diglycerides, phospholipids, and others. The functions of lipids include storing ...
s, steroids are highly concentrated energy stores. However, they are not typically sources of energy; in mammals, they are normally metabolized and excreted.
Steroids play critical roles in a number of disorders, including malignancies like prostate cancer, where steroid production inside and outside the tumour promotes cancer cell aggressiveness.
Biosynthesis and metabolism
plant
Plants are the eukaryotes that form the Kingdom (biology), kingdom Plantae; they are predominantly Photosynthesis, photosynthetic. This means that they obtain their energy from sunlight, using chloroplasts derived from endosymbiosis with c ...
s are made from lanosterol
Lanosterol is a tetracyclic triterpenoid and is the compound from which all animal and fungal steroids are derived. By contrast, plant steroids are produced via cycloartenol. In the eyes of vertebrates, lanosterol is a natural constituent, havin ...
(in animals and fungi; see examples above) or cycloartenol
Cycloartenol is an important triterpenoid often found in plants. It belongs to the sterol class of steroids. It is the starting point for the synthesis of almost all plant steroids, making them chemically distinct from the steroids of fungi and a ...
(in other eukaryotes). Both lanosterol and cycloartenol derive from cyclization
A cyclic compound (or ring compound) is a term for a compound in the field of chemistry in which one or more series of atoms in the compound is connected to form a ring. Rings may vary in size from three to many atoms, and include examples where ...
of the triterpene, triterpenoid squalene
Squalene is an organic compound. It is a triterpene with the formula C30H50. It is a colourless oil, although impure samples appear yellow. It was originally obtained from shark liver oil (hence its name, as '' Squalus'' is a genus of sharks). ...
. Lanosterol and cycloartenol are sometimes called protosterols because they serve as the starting compounds for all other steroids.
Steroid biosynthesis is an anabolism, anabolic pathway which produces steroids from simple precursors. A unique biosynthetic pathway is followed in animals (compared to many other organisms), making the pathway a common target for antibiotics and other anti-infection drugs. Steroid metabolism in humans is also the target of cholesterol-lowering drugs, such as statins. In humans and other animals the biosynthesis of steroids follows the mevalonate pathway, which uses acetyl-CoA as building blocks for dimethylallyl pyrophosphate, dimethylallyl diphosphate (DMAPP) and isopentenyl pyrophosphate, isopentenyl diphosphate (IPP).
In subsequent steps DMAPP and IPP conjugate to form Farnesyl pyrophosphate, farnesyl diphosphate (FPP), which further conjugates with each other to form the linear triterpenoid squalene. Squalene biosynthesis is catalyzed by Farnesyl-diphosphate farnesyltransferase, squalene synthase, which belongs to the squalene/phytoene synthase family. Subsequent Epoxide, epoxidation and cyclization of squalene generate lanosterol, which is the starting point for additional modifications into other steroids (steroidogenesis). In other eukaryotes, the cyclization product of epoxidized squalene (oxidosqualene) is cycloartenol.
Mevalonate pathway
natural product
A natural product is a natural compound or substance produced by a living organism—that is, found in nature. In the broadest sense, natural products include any substance produced by life. Natural products can also be prepared by chemical s ...
s). Here, the activated isoprene units are joined to make squalene
Squalene is an organic compound. It is a triterpene with the formula C30H50. It is a colourless oil, although impure samples appear yellow. It was originally obtained from shark liver oil (hence its name, as '' Squalus'' is a genus of sharks). ...
and folded into a set of rings to make lanosterol
Lanosterol is a tetracyclic triterpenoid and is the compound from which all animal and fungal steroids are derived. By contrast, plant steroids are produced via cycloartenol. In the eyes of vertebrates, lanosterol is a natural constituent, havin ...
. Lanosterol can then be converted into other steroids, such as cholesterol and ergosterol
Ergosterol (ergosta-5,7,22-trien-3β-ol) is a mycosterol found in cell membranes of fungi and protozoa, serving many of the same functions that cholesterol serves in animal cells. Because many fungi and protozoa cannot survive without ergostero ...
.
Two classes of medication, drugs target the mevalonate pathway: statins (like rosuvastatin), which are used to reduce hypercholesterolemia, elevated cholesterol levels, and bisphosphonates (like zoledronate), which are used to treat a number of bone-degenerative diseases.
Steroidogenesis
progestogen
Progestogens, also sometimes written progestins, progestagens or gestagens, are a class of natural or synthetic steroid hormones that bind to and activate the progesterone receptors (PR). Progesterone is the major and most important progestoge ...
s, corticosteroid
Corticosteroids are a class of steroid hormones that are produced in the adrenal cortex of vertebrates, as well as the synthetic analogues of these hormones. Two main classes of corticosteroids, glucocorticoids and mineralocorticoids, are invo ...
s (corticoids), androgen
An androgen (from Greek ''andr-'', the stem of the word meaning ) is any natural or synthetic steroid hormone that regulates the development and maintenance of male characteristics in vertebrates by binding to androgen receptors. This includes ...
s, and estrogen
Estrogen (also spelled oestrogen in British English; see spelling differences) is a category of sex hormone responsible for the development and regulation of the female reproductive system and secondary sex characteristics. There are three ...
s. Human steroidogenesis of these classes occurs in a number of locations:
* Progestogens are the precursors of all other human steroids, and all human tissues which produce steroids must first convert cholesterol to pregnenolone
Pregnenolone (P5), or pregn-5-en-3β-ol-20-one, is an endogenous steroid and precursor/metabolic intermediate in the biosynthesis of most of the steroid hormones, including the progestogens, androgens, estrogens, glucocorticoids, and mineraloc ...
. This conversion is the rate-limiting step of steroid synthesis, which occurs inside the mitochondrion of the respective tissue. It is catalyzed by the mitochondrial P450scc system.
* Cortisol, corticosterone, aldosterone are produced in the adrenal cortex.
* Estradiol, estrone and progesterone are made primarily in the ovary, estriol in placenta during pregnancy, and testosterone
Testosterone is the primary male sex hormone and androgen in Male, males. In humans, testosterone plays a key role in the development of Male reproductive system, male reproductive tissues such as testicles and prostate, as well as promoting se ...
primarily in the testes (some testosterone may also be produced in the adrenal cortex).
* Estradiol is converted from testosterone directly (in males), or via the primary pathway DHEA – androstenedione – estrone and secondarily via testosterone (in females).
* Stromal cells have been shown to produce steroids in response to signaling produced by androgen-starved prostate cancer cells.
* Some neurons and glia in the central nervous system (CNS) express the enzymes required for the local synthesis of pregnenolone, progesterone, DHEA and DHEAS, de novo synthesis, ''de novo'' or from peripheral sources.
Alternative pathways
In plants and bacteria, the non-mevalonate pathway (MEP pathway) uses Pyruvic acid, pyruvate and glyceraldehyde 3-phosphate as substrates to produce IPP and DMAPP. During diseases pathways otherwise not significant in healthy humans can become utilized. For example, in one form of congenital adrenal hyperplasia a congenital adrenal hyperplasia due to 21-hydroxylase deficiency, deficiency in the 21-hydroxylase enzymatic pathway leads to an excess of 17α-Hydroxyprogesterone (17-OHP) – this pathological excess of 17-OHP in turn may be converted to dihydrotestosterone (DHT, a potent androgen) through among others CYP17A1, 17,20 Lyase (a member of the cytochrome P450 family of enzymes), 5α-Reductase and 3α-Hydroxysteroid dehydrogenase.Catabolism and excretion
Steroids are primarily oxidized by cytochrome P450, cytochrome P450 oxidase enzymes, such as CYP3A4. These reactions introduce oxygen into the steroid ring, allowing the cholesterol to be broken up by other enzymes into bile acids. These acids can then be eliminated by secretion from the liver inbile
Bile (from Latin ''bilis''), also known as gall, is a yellow-green/misty green fluid produced by the liver of most vertebrates that aids the digestion of lipids in the small intestine. In humans, bile is primarily composed of water, is pro ...
. The expression of the oxidase gene can be Downregulation and upregulation, upregulated by the steroid sensor PXR when there is a high blood concentration of steroids. Steroid hormones, lacking the side chain of cholesterol and bile acids, are typically Hydroxylation, hydroxylated at various ring positions or 17-ketosteroid, oxidized at the 17 position, Conjugated system, conjugated with sulfate or glucuronic acid and excreted in the urine.
Isolation, structure determination, and methods of analysis
Steroid ''isolation'', depending on context, is the isolation of chemical matter required for chemical structure elucidation, derivitzation or degradation chemistry, biological testing, and other research needs (generally milligrams to grams, but often more or the isolation of "analytical quantities" of the substance of interest (where the focus is on identifying and quantifying the substance (for example, in biological tissue or fluid). The amount isolated depends on the analytical method, but is generally less than one microgram. The methods of isolation to achieve the two scales of product are distinct, but include extraction (chemistry), extraction, precipitation, adsorption, chromatography, and crystallization. In both cases, the isolated substance is purified to chemical homogeneity; combined separation and analytical methods, such as Liquid chromatography–mass spectrometry, LC-MS, are chosen to be "orthogonal"—achieving their separations based on distinct modes of interaction between substance and isolating matrix—to detect a single species in the pure sample. ''Structure determination'' refers to the methods to determine the chemical structure of an isolated pure steroid, using an evolving array of chemical and physical methods which have included NMR and small-molecule crystallography. ''Methods of analysis'' overlap both of the above areas, emphasizing analytical methods to determining if a steroid is present in a mixture and determining its quantity.Chemical synthesis
Microbial catabolism ofphytosterol
Phytosterols are phytosteroids, similar to cholesterol, that serve as structural components of biological membranes of plants. They encompass plant sterols and stanol ester, stanols. More than 250 sterols and related compounds have been identified ...
side chain
In organic chemistry and biochemistry, a side chain is a substituent, chemical group that is attached to a core part of the molecule called the "main chain" or backbone chain, backbone. The side chain is a hydrocarbon branching element of a mo ...
s yields C-19 steroids, C-22 steroids, and 17-ketosteroids (i.e. Precursor (chemistry), precursors to adrenocortical hormones and contraceptives). The addition and modification of functional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
s is key when producing the wide variety of medications available within this chemical classification. These modifications are performed using conventional organic synthesis and/or biotransformation techniques.
Precursors
Semisynthesis
The semisynthesis of steroids often begins from precursors such ascholesterol
Cholesterol is the principal sterol of all higher animals, distributed in body Tissue (biology), tissues, especially the brain and spinal cord, and in Animal fat, animal fats and oils.
Cholesterol is biosynthesis, biosynthesized by all anima ...
, phytosterol
Phytosterols are phytosteroids, similar to cholesterol, that serve as structural components of biological membranes of plants. They encompass plant sterols and stanol ester, stanols. More than 250 sterols and related compounds have been identified ...
s, or sapogenins. The efforts of Syntex, a company involved in the Mexican barbasco trade, used ''Dioscorea mexicana'' to produce the sapogenin diosgenin in the early days of the synthetic steroid Fine chemical#Pharmaceuticals, pharmaceutical industry.
Total synthesis
Some steroidal hormones are economically obtained only by total synthesis from petrochemicals (e.g. 13-alkyl steroids). For example, the pharmaceutical Norgestrel begins from methoxy-1-tetralone, a petrochemical derived from phenol.Research awards
A number of Nobel Prizes have been awarded for steroid research, including: * 1927 (Nobel Prize in Chemistry, Chemistry) Heinrich Otto Wieland — Constitution of bile acids and sterols and their connection to vitamins * 1928 (Chemistry) Adolf Otto Reinhold Windaus — Constitution of sterols and their connection to vitamins * 1939 (Chemistry) Adolf Butenandt and Leopold Ruzicka, Leopold Ružička — Isolation and structural studies of steroid sex hormones, and related studies on higher terpenes * 1950 (Nobel Prize in Physiology or Medicine, Physiology or Medicine) Edward Calvin Kendall, Tadeus Reichstein, and Philip Hench — Structure and biological effects of adrenocortical hormone, adrenal hormones * 1965 (Chemistry) Robert Burns Woodward — In part, for the synthesis of cholesterol, cortisone, andlanosterol
Lanosterol is a tetracyclic triterpenoid and is the compound from which all animal and fungal steroids are derived. By contrast, plant steroids are produced via cycloartenol. In the eyes of vertebrates, lanosterol is a natural constituent, havin ...
* 1969 (Chemistry) Derek Barton and Odd Hassel — Development of the concept of conformation in chemistry, emphasizing the steroid nucleus
* 1975 (Chemistry) Vladimir Prelog — In part, for developing methods to determine the stereochemical course of cholesterol biosynthesis from mevalonic acid via squalene
Squalene is an organic compound. It is a triterpene with the formula C30H50. It is a colourless oil, although impure samples appear yellow. It was originally obtained from shark liver oil (hence its name, as '' Squalus'' is a genus of sharks). ...
See also
* Adrenal gland * Batrachotoxin * List of steroid abbreviations * List of steroids * Membrane steroid receptor * Pheromone * Reverse cholesterol transport * Steroidogenesis inhibitor * Steroidogenic acute regulatory protein * Steroidogenic enzymeReferences
Bibliography
* * * A concise history of the study of steroids. * A review of the history of steroid synthesis, especially biomimetic. * Adrenal steroidogenesis pathway. * * {{Authority control Steroids, Wikipedia articles with sections published in WikiJournal of Medicine Polycyclic organic compounds