Standard enthalpy change of fusion on:
[Wikipedia]
[Google]
[Amazon]
 In
In
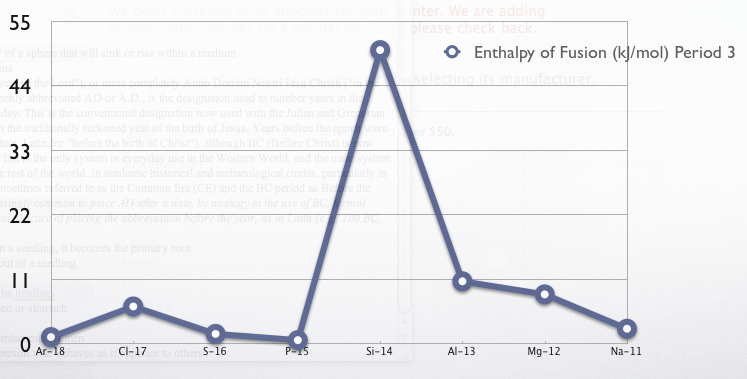
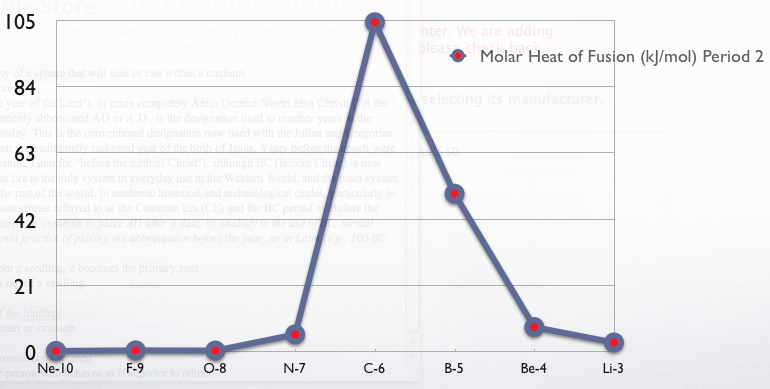 {, class="wikitable sortable"
, -
! rowspan=2, Substance
! colspan=2, Heat of fusion
, -
! (cal/g)
! (J/g)
, -
,
{, class="wikitable sortable"
, -
! rowspan=2, Substance
! colspan=2, Heat of fusion
, -
! (cal/g)
! (J/g)
, -
,
thermodynamics
Thermodynamics is a branch of physics that deals with heat, Work (thermodynamics), work, and temperature, and their relation to energy, entropy, and the physical properties of matter and radiation. The behavior of these quantities is governed b ...
, the enthalpy of fusion of a substance, also known as (latent) heat of fusion, is the change in its enthalpy
Enthalpy () is the sum of a thermodynamic system's internal energy and the product of its pressure and volume. It is a state function in thermodynamics used in many measurements in chemical, biological, and physical systems at a constant extern ...
resulting from providing energy
Energy () is the physical quantity, quantitative physical property, property that is transferred to a physical body, body or to a physical system, recognizable in the performance of Work (thermodynamics), work and in the form of heat and l ...
, typically heat
In thermodynamics, heat is energy in transfer between a thermodynamic system and its surroundings by such mechanisms as thermal conduction, electromagnetic radiation, and friction, which are microscopic in nature, involving sub-atomic, ato ...
, to a specific quantity of the substance to change its state
State most commonly refers to:
* State (polity), a centralized political organization that regulates law and society within a territory
**Sovereign state, a sovereign polity in international law, commonly referred to as a country
**Nation state, a ...
from a solid
Solid is a state of matter where molecules are closely packed and can not slide past each other. Solids resist compression, expansion, or external forces that would alter its shape, with the degree to which they are resisted dependent upon the ...
to a liquid
Liquid is a state of matter with a definite volume but no fixed shape. Liquids adapt to the shape of their container and are nearly incompressible, maintaining their volume even under pressure. The density of a liquid is usually close to th ...
, at constant pressure.
The enthalpy of fusion is the amount of energy required to convert one mole of solid into liquid. For example, when melting
Melting, or fusion, is a physical process that results in the phase transition of a substance from a solid to a liquid. This occurs when the internal energy of the solid increases, typically by the application of heat or pressure, which inc ...
1 kg of ice (at 0 °C under a wide range of pressures), 333.55 kJ of energy is absorbed with no temperature
Temperature is a physical quantity that quantitatively expresses the attribute of hotness or coldness. Temperature is measurement, measured with a thermometer. It reflects the average kinetic energy of the vibrating and colliding atoms making ...
change. The heat of solidification (when a substance changes from liquid to solid) is equal and opposite.
This energy includes the contribution required to make room for any associated change in volume by displacing its environment against ambient pressure. The temperature at which the phase transition
In physics, chemistry, and other related fields like biology, a phase transition (or phase change) is the physical process of transition between one state of a medium and another. Commonly the term is used to refer to changes among the basic Sta ...
occurs is the melting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state of matter, state from solid to liquid. At the melting point the solid and liquid phase (matter), phase exist in Thermodynamic equilib ...
or the freezing point, according to context. By convention, the pressure is assumed to be unless otherwise specified.
Overview
The enthalpy of fusion is alatent heat
Latent heat (also known as latent energy or heat of transformation) is energy released or absorbed, by a body or a thermodynamic system, during a constant-temperature process—usually a first-order phase transition, like melting or condensation. ...
, because, while melting, the heat energy needed to change the substance from solid to liquid does not cause any increase in temperature. Temperature remains constant during the freezing or melting process, and only begins to change again (assuming the energy input or removal (cooling) continues) after the phase change is complete. The latent heat of fusion is the enthalpy change of any amount of substance when it melts. When the heat of fusion is referenced to a unit of mass, it is usually called the specific heat of fusion, while the molar heat of fusion refers to the enthalpy change per amount of substance
In chemistry, the amount of substance (symbol ) in a given sample of matter is defined as a ratio () between the particle number, number of elementary entities () and the Avogadro constant (). The unit of amount of substance in the International ...
in moles.
The liquid phase has a higher internal energy than the solid phase. This means energy must be supplied to a solid in order to melt it and energy is released from a liquid when it freezes, because the molecule
A molecule is a group of two or more atoms that are held together by Force, attractive forces known as chemical bonds; depending on context, the term may or may not include ions that satisfy this criterion. In quantum physics, organic chemi ...
s in the liquid experience weaker intermolecular force
An intermolecular force (IMF; also secondary force) is the force that mediates interaction between molecules, including the electromagnetic forces of attraction
or repulsion which act between atoms and other types of neighbouring particles (e.g. ...
s and so have a higher potential energy (a kind of bond-dissociation energy
The bond-dissociation energy (BDE, ''D''0, or ''DH°'') is one measure of the strength of a chemical bond . It can be defined as the standard enthalpy change when is cleaved by homolysis to give fragments A and B, which are usually radical s ...
for intermolecular forces).
When liquid water is cooled, its temperature falls steadily until it drops just below the line of freezing point at 0 °C. The temperature then remains constant at the freezing point while the water crystallizes. Once the water is completely frozen, its temperature resumes a colder trend.
The enthalpy of fusion is almost always a positive quantity; helium
Helium (from ) is a chemical element; it has chemical symbol, symbol He and atomic number 2. It is a colorless, odorless, non-toxic, inert gas, inert, monatomic gas and the first in the noble gas group in the periodic table. Its boiling point is ...
is the only known exception. Helium-3
Helium-3 (3He see also helion) is a light, stable isotope of helium with two protons and one neutron. (In contrast, the most common isotope, helium-4, has two protons and two neutrons.) Helium-3 and hydrogen-1 are the only stable nuclides with ...
has a negative enthalpy of fusion at temperatures below 0.3 K. Helium-4
Helium-4 () is a stable isotope of the element helium. It is by far the more abundant of the two naturally occurring isotopes of helium, making up about 99.99986% of the helium on Earth. Its nucleus is identical to an alpha particle, and consi ...
also has a very slightly negative enthalpy of fusion below . This means that, at appropriate constant pressures, these substances freeze with the addition of heat. In the case of 4He, this pressure range is between 24.992 and .
 {, class="wikitable sortable"
, -
! rowspan=2, Substance
! colspan=2, Heat of fusion
, -
! (cal/g)
! (J/g)
, -
,
{, class="wikitable sortable"
, -
! rowspan=2, Substance
! colspan=2, Heat of fusion
, -
! (cal/g)
! (J/g)
, -
, water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known liv ...
, 79.72
, 333.55
, -
, methane
Methane ( , ) is a chemical compound with the chemical formula (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas. The abundance of methane on Earth makes ...
, 13.96
, 58.99
, -
, propane
Propane () is a three-carbon chain alkane with the molecular formula . It is a gas at standard temperature and pressure, but becomes liquid when compressed for transportation and storage. A by-product of natural gas processing and petroleum ref ...
, 19.11
, 79.96
, -
, glycerol
Glycerol () is a simple triol compound. It is a colorless, odorless, sweet-tasting, viscous liquid. The glycerol backbone is found in lipids known as glycerides. It is also widely used as a sweetener in the food industry and as a humectant in pha ...
, 47.95
, 200.62
, -
, formic acid
Formic acid (), systematically named methanoic acid, is the simplest carboxylic acid. It has the chemical formula HCOOH and structure . This acid is an important intermediate in chemical synthesis and occurs naturally, most notably in some an ...
, 66.05
, 276.35
, -
, acetic acid
Acetic acid , systematically named ethanoic acid , is an acidic, colourless liquid and organic compound with the chemical formula (also written as , , or ). Vinegar is at least 4% acetic acid by volume, making acetic acid the main compone ...
, 45.90
, 192.09
, -
, acetone
Acetone (2-propanone or dimethyl ketone) is an organic compound with the chemical formula, formula . It is the simplest and smallest ketone (). It is a colorless, highly Volatile organic compound, volatile, and flammable liquid with a charact ...
, 23.42
, 97.99
, -
, benzene
Benzene is an Organic compound, organic chemical compound with the Chemical formula#Molecular formula, molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar hexagonal Ring (chemistry), ring with one hyd ...
, 30.45
, 127.40
, -
, myristic acid
Myristic acid (IUPAC name: tetradecanoic acid) is a common saturated fatty acid with the molecular formula . Its salts and esters are commonly referred to as myristates or tetradecanoates. The name of the acyl group derived from myristic acid is m ...
, 47.49
, 198.70
, -
, palmitic acid
Palmitic acid (hexadecanoic acid in IUPAC nomenclature) is a fatty acid with a 16-carbon chain. It is the most common saturated fatty acid found in animals, plants and microorganisms.Gunstone, F. D., John L. Harwood, and Albert J. Dijkstra. The ...
, 39.18
, 163.93
, -
, sodium acetate
Sodium acetate, CH3COONa, also abbreviated Sodium, NaOxygen, OAcetyl, Ac, is the sodium Salt (chemistry), salt of acetic acid. This salt is colorless, deliquescent, and hygroscopy, hygroscopic.
Applications
Biotechnological
Sodium acetate is u ...
/H2O
,
, 264–289 Page 155in:
, -
, sodium sulfate
Sodium sulfate (also known as sodium sulphate or sulfate of soda) is the inorganic compound with formula Na2SO4 as well as several related hydrates. All forms are white solids that are highly soluble in water. With an annual production of 6 mill ...
/H2O
,
, 254
, -
, stearic acid
Stearic acid ( , ) is a saturated fatty acid with an 18-carbon chain. The IUPAC name is octadecanoic acid. It is a soft waxy solid with the formula . The triglyceride derived from three molecules of stearic acid is called stearin. Stearic acid ...
, 47.54
, 198.91
, -
, gallium
Gallium is a chemical element; it has Chemical symbol, symbol Ga and atomic number 31. Discovered by the French chemist Paul-Émile Lecoq de Boisbaudran in 1875,
elemental gallium is a soft, silvery metal at standard temperature and pressure. ...
, 19.2
, 80.4
, -
, paraffin wax
Paraffin wax (or petroleum wax) is a soft colorless solid derived from petroleum, coal, or oil shale that consists of a mixture of hydrocarbon molecules containing between 20 and 40 carbon atoms. It is solid at room temperature and melting poi ...
(C25H52)
, 47.8–52.6
, 200–220
These values are mostly from the CRC ''Handbook of Chemistry and Physics'', 62nd edition. The conversion between cal/g and J/g in the above table uses the thermochemical calorie
The calorie is a unit of energy that originated from the caloric theory of heat. The large calorie, food calorie, dietary calorie, kilocalorie, or kilogram calorie is defined as the amount of heat needed to raise the temperature of one liter o ...
(calth) = 4.184 joules rather than the International Steam Table calorie (calINT) = 4.1868 joules.
Examples
Solubility prediction
The heat of fusion can also be used to predictsolubility
In chemistry, solubility is the ability of a chemical substance, substance, the solute, to form a solution (chemistry), solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form su ...
for solids in liquids. Provided an ideal solution is obtained the mole fraction
In chemistry, the mole fraction or molar fraction, also called mole proportion or molar proportion, is a quantity defined as the ratio between the amount of a constituent substance, ''ni'' (expressed in unit of moles, symbol mol), and the to ...
of solute at saturation is a function of the heat of fusion, the melting point
The melting point (or, rarely, liquefaction point) of a substance is the temperature at which it changes state of matter, state from solid to liquid. At the melting point the solid and liquid phase (matter), phase exist in Thermodynamic equilib ...
of the solid and the temperature
Temperature is a physical quantity that quantitatively expresses the attribute of hotness or coldness. Temperature is measurement, measured with a thermometer. It reflects the average kinetic energy of the vibrating and colliding atoms making ...
of the solution:
Here, is the gas constant
The molar gas constant (also known as the gas constant, universal gas constant, or ideal gas constant) is denoted by the symbol or . It is the molar equivalent to the Boltzmann constant, expressed in units of energy per temperature increment p ...
. For example, the solubility of paracetamol
Paracetamol, or acetaminophen, is a non-opioid analgesic and antipyretic agent used to treat fever and mild to moderate pain. It is a widely available over-the-counter drug sold under various brand names, including Tylenol and Panadol.
Parac ...
in water at 298 K is predicted to be:
Since the molar mass of water and paracetamol are and and the density of the solution is , an estimate of the solubility in grams per liter is:
*
*1000 g/L * (mol/18.0153g) is an estimate of the number of moles of molecules in 1L solution, using water density as a reference;
*0.0248 * (1000 g/L * (mol/18.0153g)) is the molar fraction of substance in saturated solution with a unit of mol/L;
*0.0248 * (1000 g/L * (mol/18.0153g)) * 151.17g/mol is the solute's molar fraction equivalent mass conversion;
*1-0.0248 will be the fraction of the solution that is solvent.
which is a deviation from the real solubility (240 g/L) of 11%. This error can be reduced when an additional heat capacity
Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit change in its temperature. The SI unit of heat capacity is joule per kelvin (J/K).
Heat capacity is a ...
parameter is taken into account.
Proof
At equilibrium thechemical potential
In thermodynamics, the chemical potential of a Chemical specie, species is the energy that can be absorbed or released due to a change of the particle number of the given species, e.g. in a chemical reaction or phase transition. The chemical potent ...
s for the solute in the solution and pure solid are identical:
or
with the gas constant
The molar gas constant (also known as the gas constant, universal gas constant, or ideal gas constant) is denoted by the symbol or . It is the molar equivalent to the Boltzmann constant, expressed in units of energy per temperature increment p ...
and the temperature
Temperature is a physical quantity that quantitatively expresses the attribute of hotness or coldness. Temperature is measurement, measured with a thermometer. It reflects the average kinetic energy of the vibrating and colliding atoms making ...
.
Rearranging gives:
and since
the heat of fusion being the difference in chemical potential between the pure liquid and the pure solid, it follows that
Application of the Gibbs–Helmholtz equation
The Gibbs–Helmholtz equation is a thermodynamic equation used to calculate changes in the Gibbs free energy of a system as a function of temperature. It was originally presented in an 1882 paper entitled " Die Thermodynamik chemischer Vorgänge" ...
:
ultimately gives:
or:
and with integration:
the result is obtained:
See also
*Enthalpy of vaporization
In thermodynamics, the enthalpy of vaporization (symbol ), also known as the (latent) heat of vaporization or heat of evaporation, is the amount of energy (enthalpy) that must be added to a liquid substance to transform a quantity of that sub ...
* Heat capacity
Heat capacity or thermal capacity is a physical property of matter, defined as the amount of heat to be supplied to an object to produce a unit change in its temperature. The SI unit of heat capacity is joule per kelvin (J/K).
Heat capacity is a ...
* Thermodynamic databases for pure substances
Thermodynamic databases contain information about List of thermodynamic properties, thermodynamic properties for substances, the most important being enthalpy, entropy, and Gibbs free energy. Numerical values of these thermodynamic properties are ...
* Joback method (Estimation of the heat of fusion from molecular structure)
* Latent heat
Latent heat (also known as latent energy or heat of transformation) is energy released or absorbed, by a body or a thermodynamic system, during a constant-temperature process—usually a first-order phase transition, like melting or condensation. ...
* Lattice energy
In chemistry, the lattice energy is the energy change (released) upon formation of one mole of a crystalline compound from its infinitely separated constituents, which are assumed to initially be in the gaseous state at 0 K. It is a measure of ...
* Heat of dilution
Notes
References
* * {{DEFAULTSORT:Enthalpy Of Fusion Enthalpy