Spin Chemistry on:
[Wikipedia]
[Google]
[Amazon]
Spin chemistry is a sub-field of
 A radical is a molecule with an odd number of
A radical is a molecule with an odd number of

 The Zeeman interaction is an interaction between spin and external magnetic field, and is given by the equation
:
where is the energy of the Zeeman interaction, is the Larmor frequency, is the external magnetic field, is the
The Zeeman interaction is an interaction between spin and external magnetic field, and is given by the equation
:
where is the energy of the Zeeman interaction, is the Larmor frequency, is the external magnetic field, is the
Spin Chemistry Community website
chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules a ...
positioned at the intersection of chemical kinetics
Chemical kinetics, also known as reaction kinetics, is the branch of physical chemistry that is concerned with understanding the rates of chemical reactions. It is different from chemical thermodynamics, which deals with the direction in which a ...
, photochemistry
Photochemistry is the branch of chemistry concerned with the chemical effects of light. Generally, this term is used to describe a chemical reaction caused by absorption of ultraviolet (wavelength from 100 to 400 Nanometre, nm), visible ligh ...
, magnetic resonance and free radical
A daughter category of ''Ageing'', this category deals only with the biological aspects of ageing.
Ageing
Biogerontology
Biological processes
Causes of death
Cellular processes
Gerontology
Life extension
Metabolic disorders
Metabolism
...
chemistry, that deals with magnetic and spin effects in chemical reactions. Spin chemistry concerns phenomena such as chemically induced dynamic nuclear polarization (CIDNP), chemically induced electron polarization (CIDEP), magnetic isotope
Isotopes are distinct nuclear species (or ''nuclides'') of the same chemical element. They have the same atomic number (number of protons in their Atomic nucleus, nuclei) and position in the periodic table (and hence belong to the same chemica ...
effects in chemical reactions, and it is hypothesized to be key in the underlying mechanism for avian magnetoreception and consciousness.
Radical-pair mechanism
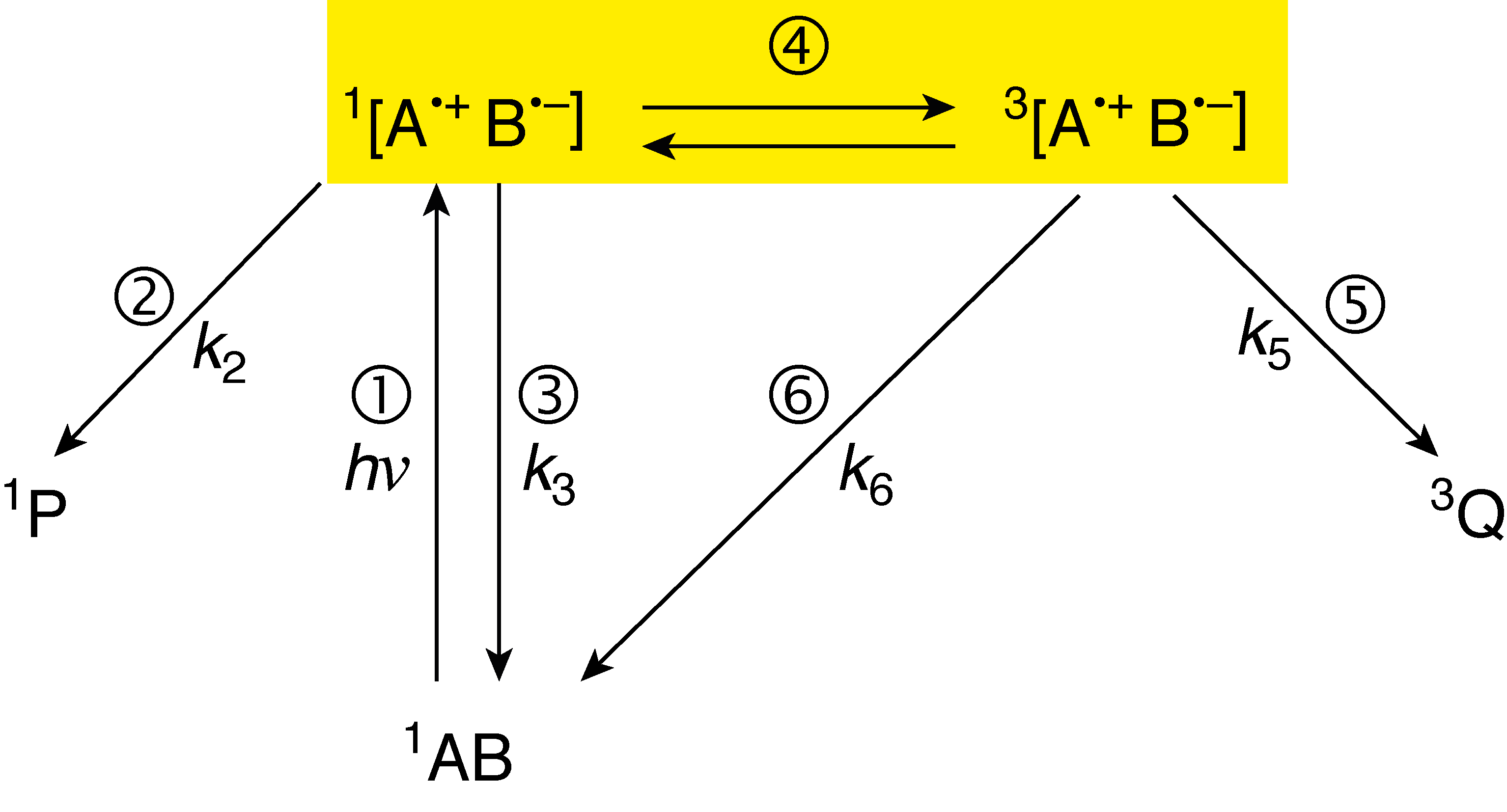
The radical-pair mechanism explains how a magnetic field can affect reaction kinetics by affecting electron spin dynamics. Most commonly demonstrated in reactions of organic compounds involving radical intermediates, a magnetic field can speed up a reaction by decreasing the frequency of reverse reactions.History
The radical-pair mechanism emerged as an explanation to CIDNP and CIDEP and was proposed in 1969 by Closs; Kaptein and Oosterhoff.Radicals and radical-pairs
electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
s, and is induced in a variety of ways, including ultraviolet radiation. A sun burn is largely due to radical formation from this radiation. The radical-pair, however, is not simply two radicals. This is because radical-pairs (specifically singlets) are quantum entangled, even as separate molecules. More fundamental to the radical-pair mechanism, however, is the fact that radical-pair electrons both have spin, short for spin angular momentum, which gives each separate radical a magnetic moment
In electromagnetism, the magnetic moment or magnetic dipole moment is the combination of strength and orientation of a magnet or other object or system that exerts a magnetic field. The magnetic dipole moment of an object determines the magnitude ...
. Therefore, spin states can be altered by magnetic fields.
Singlet and triplet spin states
The radical-pair is characterized as triplet or singlet by the spin state of the ''two'' lone electrons, paired together. The spin relationship is such that the two unpaired electrons, one in each radical molecule, may have opposite spin (singlet; anticorrelated), or the same spin (triplet; correlated). The singlet state is called such because there is only one way for the electrons’ spins to anticorrelate (S), whereas the triplet state is called such because the electron's spin may be correlated in three different fashions, denoted T+1, T0, and T−1.
Reaction kinetics and the Zeeman interaction
Spin states relate to chemical and biochemical reaction mechanisms because bonds can be formed only between two electrons of opposite spin ( Hund's rules). Sometimes when a bond is broken in a particular manner, for example, when struck by photons, each electron in the bond relocates to each respective molecule, and a radical-pair is formed. Furthermore, the spin of each electron previously involved in the bond is conserved, which means that the radical-pair now formed is a singlet (each electron has opposite spin, as in the origin bond). As such, the reverse reaction, i.e. the reforming of a bond, called recombination, readily occurs. The radical-pair mechanism explains how external magnetic fields can prevent radical-pair recombination with Zeeman interactions, the interaction between spin and an external magnetic field, and shows how a higher occurrence of the triplet state accelerates radical reactions because triplets can proceed only to products, and singlets are in equilibrium with the reactants as well as with the products. Zeeman interactions can "flip" only one of the radical's electron's spin if the radical-pair isanisotropic
Anisotropy () is the structural property of non-uniformity in different directions, as opposed to isotropy. An anisotropic object or pattern has properties that differ according to direction of measurement. For example, many materials exhibit ver ...
, thereby converting singlet radical-pairs to triplets.
 The Zeeman interaction is an interaction between spin and external magnetic field, and is given by the equation
:
where is the energy of the Zeeman interaction, is the Larmor frequency, is the external magnetic field, is the
The Zeeman interaction is an interaction between spin and external magnetic field, and is given by the equation
:
where is the energy of the Zeeman interaction, is the Larmor frequency, is the external magnetic field, is the Bohr magneton
In atomic physics, the Bohr magneton (symbol ) is a physical constant and the natural unit for expressing the magnetic moment of an electron caused by its orbital or spin angular momentum.
In SI units, the Bohr magneton is defined as
\mu_\mat ...
, is the Planck constant
The Planck constant, or Planck's constant, denoted by h, is a fundamental physical constant of foundational importance in quantum mechanics: a photon's energy is equal to its frequency multiplied by the Planck constant, and the wavelength of a ...
, and is the ''g''-factor of a free electron, which is slightly different in different radicals.
It is common to see the Zeeman interaction formulated in other ways.
Hyperfine interactions
Hyperfine interactions, the internal magnetic fields of local magnetic isotopes, play a significant role as well in the spin dynamics of radical-pairs.Zeeman interactions and magnetoreception
Because the Zeeman interaction is a function of magnetic field and Larmor frequency, it can be obstructed or amplified by altering the external magnetic or the Larmor frequency with experimental instruments that generate oscillating fields. It has been observed that migratory birds lose their navigational abilities in such conditions where the Zeeman interaction is obstructed in radical-pairs.External links
Spin Chemistry Community website
References
{{DEFAULTSORT:Spin Chemistry Physical chemistry Nuclear magnetic resonance