Shapiro reaction on:
[Wikipedia]
[Google]
[Amazon]
The Shapiro reaction or tosylhydrazone decomposition is an 
 The reaction's directionality is controlled by the stereochemistry of the hydrazone, with deprotonation occurring cis to the tosylamide group. This is due to coordination by the nitrogen atom.
The reaction's directionality is controlled by the stereochemistry of the hydrazone, with deprotonation occurring cis to the tosylamide group. This is due to coordination by the nitrogen atom.
 Importantly, the Shapiro reaction cannot be used to synthesize 1-lithioalkenes (and the resulting functionalized derivatives), as sulfonylhydrazones derived from aldehydes undergo exclusive addition of the organolithium base to the carbon of the C–N double bond.
Importantly, the Shapiro reaction cannot be used to synthesize 1-lithioalkenes (and the resulting functionalized derivatives), as sulfonylhydrazones derived from aldehydes undergo exclusive addition of the organolithium base to the carbon of the C–N double bond.


organic reaction
Organic reactions are chemical reactions involving organic compounds. The basic organic chemistry reaction types are addition reactions, elimination reactions, substitution reactions, pericyclic reactions, rearrangement reactions, mechanistic organ ...
in which a ketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
or aldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
is converted to an alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
through an intermediate hydrazone
Hydrazones are a class of organic compounds with the structure . They are related to ketones and aldehydes by the replacement of the oxygen =O with the = functional group. They are formed usually by the action of hydrazine on ketones or aldehydes. ...
in the presence of 2 equivalents of organolithium reagent
In organometallic chemistry, organolithium reagents are chemical compounds that contain carbon–lithium (C–Li) bonds. These reagents are important in organic synthesis, and are frequently used to transfer the organic group or the lithium atom ...
. The reaction was discovered by Robert H. Shapiro in 1967. The Shapiro reaction was used in the Nicolaou Taxol total synthesis. This reaction is very similar to the Bamford–Stevens reaction, which also involves the basic decomposition of tosyl hydrazones.
Reaction mechanism and directionality
In a prelude to the actual Shapiro reaction, aketone
In organic chemistry, a ketone is an organic compound with the structure , where R and R' can be a variety of carbon-containing substituents. Ketones contain a carbonyl group (a carbon-oxygen double bond C=O). The simplest ketone is acetone ( ...
or an aldehyde
In organic chemistry, an aldehyde () (lat. ''al''cohol ''dehyd''rogenatum, dehydrogenated alcohol) is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred ...
is converted to the tosylhydrazone. Two equivalents of ''n''-butyllithium gives the carbanion, which produces a carbon–carbon double bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betw ...
, ejecting the tosyl
In organic chemistry, a toluenesulfonyl group (tosyl group, abbreviated Ts or TosIn this article, "Ts", unless otherwise stated, means tosyl, not tennessine.) is a univalent functional group with the chemical formula . It consists of a tolyl ...
anion. The resulting diazonium anion loses molecular nitrogen giving the vinyllithium species.
: The reaction's directionality is controlled by the stereochemistry of the hydrazone, with deprotonation occurring cis to the tosylamide group. This is due to coordination by the nitrogen atom.
The reaction's directionality is controlled by the stereochemistry of the hydrazone, with deprotonation occurring cis to the tosylamide group. This is due to coordination by the nitrogen atom.
Scope
The position of the alkene in the product is controlled by the site of deprotonation by the organolithium base. In general, the kinetically favored, less substituted site of differentially substituted tosylhydrazones is deprotonated selectively, leading to the less substituted vinyllithium intermediate. Although many secondary reactions exist for the vinyllithiumfunctional group
In organic chemistry, a functional group is any substituent or moiety (chemistry), moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions r ...
, in the Shapiro reaction in particular water
Water is an inorganic compound with the chemical formula . It is a transparent, tasteless, odorless, and Color of water, nearly colorless chemical substance. It is the main constituent of Earth's hydrosphere and the fluids of all known liv ...
is added, resulting in protonation to the alkene
In organic chemistry, an alkene, or olefin, is a hydrocarbon containing a carbon–carbon double bond. The double bond may be internal or at the terminal position. Terminal alkenes are also known as Alpha-olefin, α-olefins.
The Internationa ...
. Other reactions of vinyllithium compounds include alkylation reactions with for instance alkyl halides.
Catalytic Shapiro reaction
Traditional Shapiro reactions require stoichiometric (sometimes excess) amounts of base to generate the alkenyllithium reagents. To combat this problem, Yamamoto and coworkers developed an efficient stereoselective and regioselective route to alkenes using a combination of ketone phenylaziridinylhydrazones as arenesulfonylhydrazone equivalents with a catalytic amount of lithium amides. The required phenylaziridinylhydrazone was prepared from the condensation of undecan-6-one with 1-amino-2-phenylaziridine. Treatment of the phenylaziridinylhydrazone with 0.3 equivalents of LDA in ether resulted in the alkene shown below with a ''cis'':''trans'' ratio of 99.4:0.6. The ratio was determined by capillary GLC analysis after conversion to the corresponding epoxides with mCPBA. The catalyst loading can be reduced to 0.05 equivalents in the case of a 30mmol scale reaction. The high stereoselectivity is obtained by the preferential abstraction of the α-methylene hydrogen syn to the phenylaziridine, and is also accounted for by the internal chelation of the lithiated intermediated.
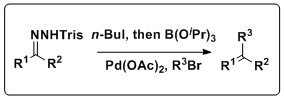
A one pot in situ combined Shapiro-Suzuki reaction
The Shapiro reaction can also be combined with theSuzuki reaction
The Suzuki reaction or Suzuki coupling is an organic reaction that uses a palladium complex catalyst to cross-couple a boronic acid to an organohalide. It was first published in 1979 by Akira Suzuki, and he shared the 2010 Nobel Prize in Chemi ...
to produce a variety of olefin products. Keay and coworkers have developed methodology that combines these reactions in a one pot process that does not require the isolation of the boronic acid, a setback of the traditional Suzuki coupling. This reaction has a wide scope, tolerating a slew of trisylhydrazones and aryl halides, as well as several solvents and Pd sources.

An application of the Shapiro reaction in total synthesis
The Shapiro reaction has been used to generate olefins towards to complex natural products. K. Mori and coworkers wanted to determine the absolute configuration of the phytocassane group of a class of natural products calledphytoalexin
Phytoalexins are antimicrobial substances, some of which are antioxidative as well. They are defined not by their having any particular chemical structure or character, but by the fact that they are defensively synthesized ''de novo'' by plants ...
s. This was accomplished by preparing the naturally occurring (–)-phytocassane D from (''R'')- Wieland-Miescher ketone. On the way to (–)-phytocassane D, a tricyclic ketone was subjected to Shapiro reaction conditions to yield the cyclic alkene product.

See also
* Hydrazone iodination * Wolff–Kishner reductionReferences
{{Alkenes Carbon-carbon bond forming reactions Olefination reactions Organic redox reactions Name reactions