Shape Control In Nanocrystal Growth on:
[Wikipedia]
[Google]
[Amazon]
Shape control in nanocrystal growth is the control of the shape of nanocrystals (crystalline
 Generally, a surface with high Miller indices has a high surface energy. Qualitatively, this follows from the fact that for higher Miller indices, on average more surface atoms are at positions at a corner instead of a terrace, as can be seen in the figure. After all, corner atoms have even fewer neighbours to interact with than terrace atoms. For example, in the case of a 2D square lattice, they have two instead of three neighbours. These additionally broken bonds all cost energy, which is why lower Miller indices planes generally have lower surface energies and are as a consequence more stable. However, the comparison is in fact somewhat more complex, as the surface energy as function of the Miller indices also depends on the structure of the
Generally, a surface with high Miller indices has a high surface energy. Qualitatively, this follows from the fact that for higher Miller indices, on average more surface atoms are at positions at a corner instead of a terrace, as can be seen in the figure. After all, corner atoms have even fewer neighbours to interact with than terrace atoms. For example, in the case of a 2D square lattice, they have two instead of three neighbours. These additionally broken bonds all cost energy, which is why lower Miller indices planes generally have lower surface energies and are as a consequence more stable. However, the comparison is in fact somewhat more complex, as the surface energy as function of the Miller indices also depends on the structure of the
nanoparticle
A nanoparticle or ultrafine particle is a particle of matter 1 to 100 nanometres (nm) in diameter. The term is sometimes used for larger particles, up to 500 nm, or fibers and tubes that are less than 100 nm in only two directions. At ...
s) formed in their synthesis
Synthesis or synthesize may refer to:
Science Chemistry and biochemistry
*Chemical synthesis, the execution of chemical reactions to form a more complex molecule from chemical precursors
**Organic synthesis, the chemical synthesis of organi ...
by means of varying reaction conditions
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an energy change as new products ...
. This is a concept studied in nanosciences, which is a part of both chemistry
Chemistry is the scientific study of the properties and behavior of matter. It is a physical science within the natural sciences that studies the chemical elements that make up matter and chemical compound, compounds made of atoms, molecules a ...
and condensed matter physics
Condensed matter physics is the field of physics that deals with the macroscopic and microscopic physical properties of matter, especially the solid and liquid State of matter, phases, that arise from electromagnetic forces between atoms and elec ...
. There are two processes involved in the growth of these nanocrystals. Firstly, volume Gibbs free energy
In thermodynamics, the Gibbs free energy (or Gibbs energy as the recommended name; symbol is a thermodynamic potential that can be used to calculate the maximum amount of Work (thermodynamics), work, other than Work (thermodynamics)#Pressure–v ...
of the system containing the nanocrystal in solution decreases as the nanocrystal size increases. Secondly, each crystal has a surface Gibbs free energy that can be minimized by adopting the shape that is energetically most favorable. Surface energies of crystal planes are related to their Miller indices
Miller indices form a notation system in crystallography for lattice planes in crystal (Bravais) lattices.
In particular, a family of lattice planes of a given (direct) Bravais lattice is determined by three integers ''h'', ''k'', and ''� ...
, which is why these can help predict the equilibrium shape of a certain nanocrystal.
Because of these two different processes, there are two competing regimes in which nanocrystal growth can take place: the kinetic regime, where the crystal growth is controlled by minimization of the volume free energy, and the thermodynamic regime, where growth is controlled by minimization of the surface free energy. High concentration, low temperatures and short aging times favor the kinetic regime, whereas low concentration, high temperatures and long aging times favor the thermodynamic regime.
The different regimes lead to different shapes of the nanocrystals: the kinetic regime can give anisotropic
Anisotropy () is the structural property of non-uniformity in different directions, as opposed to isotropy. An anisotropic object or pattern has properties that differ according to direction of measurement. For example, many materials exhibit ver ...
shapes which are often connected to the kinetic Wulff construction, whereas the thermodynamic regime gives equilibrium, isotropic
In physics and geometry, isotropy () is uniformity in all orientations. Precise definitions depend on the subject area. Exceptions, or inequalities, are frequently indicated by the prefix ' or ', hence '' anisotropy''. ''Anisotropy'' is also ...
shapes, which can be determined using the Wulff construction
The Wulff construction is a method to determine the equilibrium shape of a droplet or crystal of fixed volume inside a separate phase (usually its saturated solution or vapor). Energy minimization arguments are used to show that certain crystal ...
.
The shape of the nanocrystal determines many properties of the nanocrystal, such as the band gap
In solid-state physics and solid-state chemistry, a band gap, also called a bandgap or energy gap, is an energy range in a solid where no electronic states exist. In graphs of the electronic band structure of solids, the band gap refers to t ...
and polarization
Polarization or polarisation may refer to:
Mathematics
*Polarization of an Abelian variety, in the mathematics of complex manifolds
*Polarization of an algebraic form, a technique for expressing a homogeneous polynomial in a simpler fashion by ...
of emitted light.
Miller indices and surface energy
Thesurface energy
In surface science, surface energy (also interfacial free energy or surface free energy) quantifies the disruption of intermolecular bonds that occurs when a surface is created. In solid-state physics, surfaces must be intrinsically less energe ...
of a solid is the free energy per unit area of its surface. It equals half the energy per unit area needed for cutting a larger piece of solid in two parts along the surface under examination. This costs energy because chemical bond
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds or through the sharing of electrons a ...
s are broken. Typically, materials are considered to have one specific surface energy. However, in the case of crystal
A crystal or crystalline solid is a solid material whose constituents (such as atoms, molecules, or ions) are arranged in a highly ordered microscopic structure, forming a crystal lattice that extends in all directions. In addition, macros ...
s, the surface energy depends on the orientation of the surface with respect to the unit cell
In geometry, biology, mineralogy and solid state physics, a unit cell is a repeating unit formed by the vectors spanning the points of a lattice. Despite its suggestive name, the unit cell (unlike a unit vector
In mathematics, a unit vector i ...
. Different facets of a crystal thus often have different surface energies. This can be understood from the fact that in non-crystalline materials, the building blocks that make up the material (e.g., atoms or molecules) are spread in a homogeneous
Homogeneity and heterogeneity are concepts relating to the uniformity of a substance, process or image. A homogeneous feature is uniform in composition or character (i.e., color, shape, size, weight, height, distribution, texture, language, i ...
manner. On average, the same number of bonds needs to be broken, so the same energy per unit area is needed, to create any surface. In crystals, surfaces exhibit a periodic arrangement of particles which is dependent on their orientation. Different numbers of bonds with different bond strengths are broken in the process of creating surfaces along different planes of the material, which causes the surface energies to be different. The type of plane is most easily described using the orientation of the surface with respect to a given unit cell that is characteristic of the material.
The orientation of a plane with respect to the unit cell is most conveniently expressed in terms of Miller indices
Miller indices form a notation system in crystallography for lattice planes in crystal (Bravais) lattices.
In particular, a family of lattice planes of a given (direct) Bravais lattice is determined by three integers ''h'', ''k'', and ''� ...
. For example, the set of Miller indices (110) describes the set of parallel planes ( family of lattice planes) parallel to the z-axis and cutting the x- and the y-axis once, such that every unit cell is bisected by precisely one of those planes in the x- and y-direction.
 Generally, a surface with high Miller indices has a high surface energy. Qualitatively, this follows from the fact that for higher Miller indices, on average more surface atoms are at positions at a corner instead of a terrace, as can be seen in the figure. After all, corner atoms have even fewer neighbours to interact with than terrace atoms. For example, in the case of a 2D square lattice, they have two instead of three neighbours. These additionally broken bonds all cost energy, which is why lower Miller indices planes generally have lower surface energies and are as a consequence more stable. However, the comparison is in fact somewhat more complex, as the surface energy as function of the Miller indices also depends on the structure of the
Generally, a surface with high Miller indices has a high surface energy. Qualitatively, this follows from the fact that for higher Miller indices, on average more surface atoms are at positions at a corner instead of a terrace, as can be seen in the figure. After all, corner atoms have even fewer neighbours to interact with than terrace atoms. For example, in the case of a 2D square lattice, they have two instead of three neighbours. These additionally broken bonds all cost energy, which is why lower Miller indices planes generally have lower surface energies and are as a consequence more stable. However, the comparison is in fact somewhat more complex, as the surface energy as function of the Miller indices also depends on the structure of the crystal lattice
In crystallography, crystal structure is a description of ordered arrangement of atoms, ions, or molecules in a crystal, crystalline material. Ordered structures occur from intrinsic nature of constituent particles to form symmetric patterns that ...
(e.g., bcc or fcc) and bonds between non-next nearest neighbours play a role as well.
Experimental research on noble metal
A noble metal is ordinarily regarded as a metallic chemical element, element that is generally resistant to corrosion and is usually found in nature in its native element, raw form. Gold, platinum, and the other platinum group metals (ruthenium ...
s (copper
Copper is a chemical element; it has symbol Cu (from Latin ) and atomic number 29. It is a soft, malleable, and ductile metal with very high thermal and electrical conductivity. A freshly exposed surface of pure copper has a pinkish-orang ...
, gold
Gold is a chemical element; it has chemical symbol Au (from Latin ) and atomic number 79. In its pure form, it is a brightness, bright, slightly orange-yellow, dense, soft, malleable, and ductile metal. Chemically, gold is a transition metal ...
and silver
Silver is a chemical element; it has Symbol (chemistry), symbol Ag () and atomic number 47. A soft, whitish-gray, lustrous transition metal, it exhibits the highest electrical conductivity, thermal conductivity, and reflectivity of any metal. ...
), shows that for these materials, the surface energy is well-approximated by taking only the nearest neighbours into account. The next-nearest neighbour interactions apparently do not play a major role in these metals. Also, breaking any of the nearest neighbour bonds turns out to cost the same amount of energy. Within this approximation, the surface energy of a certain Miller indices (hkl) surface is given by
with the ratio of the number of bonds broken when making this (hkl) plane with respect to making a (111) plane, and the surface energy of the (111) plane.
For any surface of an fcc crystal, is given by
assuming . In this model, the surface energy indeed increases with higher Miller indices. This is also visible in the following table, which lists computer simulated surface energies of some planes in copper (Cu), silver (Ag) and gold (Au). Again, is the number of broken bonds between nearest neighbours created when making the surface, being 3 for the (111) plane. The surface energy indeed increases for a larger number of broken bonds and therefore larger Miller indices.
It is also possible for surfaces with high Miller indices to have a low surface energy, mainly if the unit cell contains multiple atoms. After all, Miller indices are based on the unit cell, and it is the atoms, not the unit cell, that are physically present. The choice of unit cell is up to some level arbitrary as they are constructed by the interpreter. High Miller indices planes with low surface energy can be found by searching for planes with a high density of atoms. A large density of atoms in a plane after all implies a large number of in-plane bonds and thus a small number of out-of-plane bonds that would cause the surface energy to be large. If a crystal's unit cell contains only one atom, those planes naturally correspond to the planes with low Miller indices, which is why planes with low Miller indices are usually considered to have a low surface energy.
The table below shows examples of computer simulated surface energies of (hk0) planes in a NiO crystal (with ). In this case, the unit cell has a multi-atom basis, as there are two types of atoms that make up the crystal (nickel
Nickel is a chemical element; it has symbol Ni and atomic number 28. It is a silvery-white lustrous metal with a slight golden tinge. Nickel is a hard and ductile transition metal. Pure nickel is chemically reactive, but large pieces are slo ...
and oxygen
Oxygen is a chemical element; it has chemical symbol, symbol O and atomic number 8. It is a member of the chalcogen group (periodic table), group in the periodic table, a highly reactivity (chemistry), reactive nonmetal (chemistry), non ...
). The data has been ordered by increasing surface energy. From this table, it is clearly visible that the trend between surface energy and Miller indices is not as straightforward in this case as for the noble metals discussed above.
Surface energy and Equilibrium shape
Planes with low surface energies are relatively stable and thus tend to be predominantly present in thethermodynamic equilibrium
Thermodynamic equilibrium is a notion of thermodynamics with axiomatic status referring to an internal state of a single thermodynamic system, or a relation between several thermodynamic systems connected by more or less permeable or impermeable ...
shape of a crystal. After all, in equilibrium, the free energy is minimized. However, a crystal's thermodynamic equilibrium shape typically does not only consist of planes with the lowest possible surface energy. The reason for this is that involving planes with a slightly higher surface energy can decrease the total surface area, which lowers the total energy penalty for creating the material's surface. The optimum shape in terms of free energy can be determined by the Wulff construction
The Wulff construction is a method to determine the equilibrium shape of a droplet or crystal of fixed volume inside a separate phase (usually its saturated solution or vapor). Energy minimization arguments are used to show that certain crystal ...
.
Thermodynamic versus kinetic control
The growth of crystals can be carried out under two different regimes: the thermodynamic and the kinetic regime. Research on this topic is mainly centered around nanocrystals, as their synthesis is not as straightforward as that of bulk materials and thus requires a deeper understanding of types of crystal growth. Due to the high surface-volume ratio and the resulting instability, nanocrystals most easily show the difference between the thermodynamic and kinetic regime. These concepts can however be generalized further to bulk material. A commonly used production method of nanocrystals is that of growth by monomer addition. Aseed
In botany, a seed is a plant structure containing an embryo and stored nutrients in a protective coat called a ''testa''. More generally, the term "seed" means anything that can be Sowing, sown, which may include seed and husk or tuber. Seeds ...
is formed or placed in a solution of monomers that are the building blocks of the crystal. The nanocrystal (seed) grows larger by consuming the monomer
A monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or two- or three-dimensional network in a process called polymerization.
Classification
Chemis ...
s in solution. The addition of a monomer to the crystal happens at the highest energy facet
Facets () are flat faces on geometric shapes. The organization of naturally occurring facets was key to early developments in crystallography, since they reflect the underlying symmetry of the crystal structure. Gemstones commonly have facets cu ...
of the crystal, since that is the most active site and the monomer deposition thus has the lowest activation energy
In the Arrhenius model of reaction rates, activation energy is the minimum amount of energy that must be available to reactants for a chemical reaction to occur. The activation energy (''E''a) of a reaction is measured in kilojoules per mole (k ...
there. Usually, this facet is situated at a corner of the nanoparticle. These facets however, as explained in the section above, are not the most energetically favorable position for the added monomer. Thus the monomer will, if it gets the chance to, diffuse
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical p ...
along the crystal surface to a lower energy site.
The regime in which the monomers have the time to relocate is called the thermodynamic regime, as the product is formed that is expected thermodynamically. In the kinetic regime, the addition of monomers happens so rapidly that the crystal continues growing at the corners. In this case, the formed product is not at a global minimum of the free energy, but is in a metastable
In chemistry and physics, metastability is an intermediate energetic state within a dynamical system other than the system's state of least energy.
A ball resting in a hollow on a slope is a simple example of metastability. If the ball is onl ...
anisotropic
Anisotropy () is the structural property of non-uniformity in different directions, as opposed to isotropy. An anisotropic object or pattern has properties that differ according to direction of measurement. For example, many materials exhibit ver ...
state.
Thermodynamic regime
The thermodynamic regime is characterized by relatively low growth rates. Because of these, the amount theGibbs free energy
In thermodynamics, the Gibbs free energy (or Gibbs energy as the recommended name; symbol is a thermodynamic potential that can be used to calculate the maximum amount of Work (thermodynamics), work, other than Work (thermodynamics)#Pressure–v ...
is lowered due to incorporating a new monomer is smaller than due to rearranging the surface. The former is associated with the minimization of volume Gibbs free energy, whereas the latter is associated with minimizing the surface free energy. Thus, the shape evolution is driven by minimization of surface Gibbs free energy, and therefore the equilibrium shape is the one with the lowest overall surface Gibbs free energy. This corresponds to the shape with a global minimum in Gibbs free energy, which can be obtained via the Wulff construction. From this Wulff construction, it also follows that the thermodynamic product is always symmetrical.
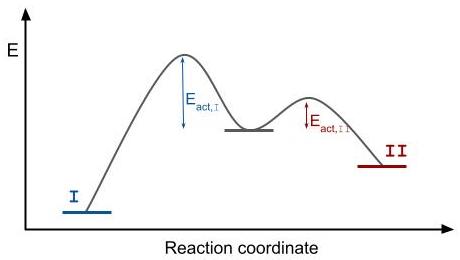
The activation energy
In the Arrhenius model of reaction rates, activation energy is the minimum amount of energy that must be available to reactants for a chemical reaction to occur. The activation energy (''E''a) of a reaction is measured in kilojoules per mole (k ...
for the thermodynamic product is higher than the activation energy for the kinetic product. From the Arrhenius equation
In physical chemistry, the Arrhenius equation is a formula for the temperature dependence of reaction rates. The equation was proposed by Svante Arrhenius in 1889, based on the work of Dutch chemist Jacobus Henricus van 't Hoff who had noted in 188 ...
with the reaction rate
The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration of a product per unit time and to the decrease in the concentration of a reactant per u ...
, '''' a constant, '''' the activation energy, the Boltzmann constant
The Boltzmann constant ( or ) is the proportionality factor that relates the average relative thermal energy of particles in a ideal gas, gas with the thermodynamic temperature of the gas. It occurs in the definitions of the kelvin (K) and the ...
and the temperature, follows that for overcoming a higher activation energy barrier, a higher temperature is needed. The thermodynamic regime is therefore associated with high temperature conditions.
The thermodynamic regime can also be characterized by giving the system a sufficiently long time to rearrange its atoms such that the global minimum in Gibbs free energy of the entire system is reached. Raising the temperature has a similar effect because the extra thermal energy
The term "thermal energy" is often used ambiguously in physics and engineering. It can denote several different physical concepts, including:
* Internal energy: The energy contained within a body of matter or radiation, excluding the potential en ...
increases the mobility of the atoms on the surface, making rearrangements easier.
Finally, the thermodynamic product can be obtained by having a low monomer concentration. This too ties into the longer time the system has at hand to rearrange before incorporating the next monomer at a lower monomer concentration, as the speed of diffusion
Diffusion is the net movement of anything (for example, atoms, ions, molecules, energy) generally from a region of higher concentration to a region of lower concentration. Diffusion is driven by a gradient in Gibbs free energy or chemical p ...
of monomers through the solution to the crystal is strongly dependent on their concentration.

Kinetic regime
The kinetic control regime is characterized by high growth rates. Due to these, the system is driven by lowering the volume Gibbs free energy, which decreases rapidly upon monomer consumption. Minimization of the surface Gibbs free energy is of less relevance to the system and the shape evolution is controlled byreaction rate
The reaction rate or rate of reaction is the speed at which a chemical reaction takes place, defined as proportional to the increase in the concentration of a product per unit time and to the decrease in the concentration of a reactant per u ...
s instead. Thus the product obtained in this regime is a metastable
In chemistry and physics, metastability is an intermediate energetic state within a dynamical system other than the system's state of least energy.
A ball resting in a hollow on a slope is a simple example of metastability. If the ball is onl ...
state, with a local minimum in Gibbs free energy.
Kinetic control is obtained when there is not enough time for atoms on the surface to diffuse to an energetically more favorable state. Conditions that favor kinetic control are low temperatures (to ensure thermal energy is smaller than activation energy of the thermodynamic reaction) and high monomer concentration (in order to obtain high growth rates). Because of the high concentrations needed for kinetic control, the diffusion spheres around the nanocrystal are small and have steep concentration gradients. The more extended parts of the crystal that reach out further through the diffusion sphere grow faster, because they reach parts of the solution where the concentration is higher. The extended facets thus grow even faster, which can result in an anisotropic product. Due to this effect, an important factor determining the final shape of the product is the shape of the initial seed.
Consequences of shape and size
Theband gap
In solid-state physics and solid-state chemistry, a band gap, also called a bandgap or energy gap, is an energy range in a solid where no electronic states exist. In graphs of the electronic band structure of solids, the band gap refers to t ...
as well as the density of states
In condensed matter physics, the density of states (DOS) of a system describes the number of allowed modes or quantum state, states per unit energy range. The density of states is defined as where N(E)\delta E is the number of states in the syste ...
of nanoparticles depend significantly on their shape and size. Generally, smaller nanoparticles have a larger band gap. Quantum confinement effects lie at the basis of this. Whereas the density of states is a smooth function for 3D crystals which are large in any direction, it becomes saw-tooth-shaped for 2D nanocrystals (e.g., disks), staircase-shaped for 1D nanocrystals (e.g., wires) and a delta function for 0D nanocrystals (balls, pyramids etc.).
Also, the polarization
Polarization or polarisation may refer to:
Mathematics
*Polarization of an Abelian variety, in the mathematics of complex manifolds
*Polarization of an algebraic form, a technique for expressing a homogeneous polynomial in a simpler fashion by ...
of emitted light and its magnetic anisotropy
In condensed matter physics, magnetic anisotropy describes how an object's magnetic properties can be anisotropy, different depending on direction. In the simplest case, there is no preferential direction for an object's magnetic moment. It will ...
are affected by the shape of the nanoparticle.
Studying different shapes of nanoparticles can improve the understanding of quantum confinement effects. By elongating an axis in certain spherical nanoparticles (quantum dot
Quantum dots (QDs) or semiconductor nanocrystals are semiconductor particles a few nanometres in size with optical and electronic properties that differ from those of larger particles via quantum mechanical effects. They are a central topic i ...
s), degeneracies in the energy levels can be resolved. Also, the energy difference between photon absorption and photon emission can be tuned using shape control. This could possibly be utilized in LED
A light-emitting diode (LED) is a semiconductor device that emits light when current flows through it. Electrons in the semiconductor recombine with electron holes, releasing energy in the form of photons. The color of the light (corresp ...
technology, as it helps to prevent re-adsorption.
References
{{reflist Nanomaterials