Serine C-palmitoyltransferase on:
[Wikipedia]
[Google]
[Amazon]
In
enzymology
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different mol ...
, a serine ''C''-palmitoyltransferase () is an enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different mol ...
that catalyzes
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
the chemical reaction
A chemical reaction is a process that leads to the chemistry, chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an Gibbs free energy, ...
:
:palmitoyl-CoA + -serine CoA + 3-dehydro--sphinganine + CO2
Thus, the two substrates of this enzyme are palmitoyl-CoA and -serine, whereas its 3 products
Product may refer to:
Business
* Product (business), an item that can be offered to a market to satisfy the desire or need of a customer.
* Product (project management), a deliverable or set of deliverables that contribute to a business solution
...
are CoA, 3-dehydro--sphinganine, and CO2. This reaction is a key step in the biosynthesis of sphingosine which is a precursor of many other sphingolipids.
This enzyme participates in sphingolipid metabolism. It employs one cofactor, pyridoxal phosphate
Pyridoxal phosphate (PLP, pyridoxal 5'-phosphate, P5P), the active form of vitamin B6, is a coenzyme in a variety of enzymatic reactions. The International Union of Biochemistry and Molecular Biology has catalogued more than 140 PLP-dependen ...
.
Nomenclature
This enzyme belongs to the family oftransferase
In biochemistry, a transferase is any one of a class of enzymes that catalyse the transfer of specific functional groups (e.g. a methyl or glycosyl group) from one molecule (called the donor) to another (called the acceptor). They are involved ...
s, specifically those acyltransferase
Acyltransferase is a type of transferase enzyme that acts upon acyl
In chemistry, an acyl group is a moiety derived by the removal of one or more hydroxyl groups from an oxoacid, including inorganic acids. It contains a double-bonded oxygen ...
s transferring groups other than aminoacyl groups. The systematic name
A systematic name is a name given in a systematic way to one unique group, organism, object or chemical substance, out of a specific population or collection. Systematic names are usually part of a nomenclature.
A semisystematic name or semitrivi ...
of this enzyme class is palmitoyl-CoA:L-serine ''C''-palmitoyltransferase (decarboxylating). Other names in common use include:
* serine palmitoyltransferase,
* SPT, 3-oxosphinganine synthetase, and
* acyl-CoA:serine C-2 acyltransferase decarboxylating.
Structure
Serine ''C''-palmitoyltransferase is a member of the AOS (a-oxoamine synthase) family of PLP-dependent enzymes, which catalyse thecondensation
Condensation is the change of the state of matter from the gas phase into the liquid phase, and is the reverse of vaporization. The word most often refers to the water cycle. It can also be defined as the change in the state of water vapor ...
of amino acids and acyl-CoA thioester substrates. The human enzyme is a heterodimer
In biochemistry, a protein dimer is a macromolecular complex or multimer formed by two protein monomers, or single proteins, which are usually non-covalently bound. Many macromolecules, such as proteins or nucleic acids, form dimers. The word ...
consisting of two monomeric subunits known as long chain base 1 and 2 (LCB1/2) encoded by separate gene
In biology, the word gene has two meanings. The Mendelian gene is a basic unit of heredity. The molecular gene is a sequence of nucleotides in DNA that is transcribed to produce a functional RNA. There are two types of molecular genes: protei ...
s. The active site of LCB2 contains lysine
Lysine (symbol Lys or K) is an α-amino acid that is a precursor to many proteins. Lysine contains an α-amino group (which is in the protonated form when the lysine is dissolved in water at physiological pH), an α-carboxylic acid group ( ...
and other key catalytic residues that are not present in LCB1, which does not participate in catalysis
Catalysis () is the increase in rate of a chemical reaction due to an added substance known as a catalyst (). Catalysts are not consumed by the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recycles quick ...
but is nevertheless required for the synthesis and stability of the enzyme.
As of late 2007, two structures have been solved for this class of enzymes, with PDB accession codes and .

Mechanism
The PLP (pyridoxal 5′-phosphate)-dependent serine ''C''-palmitoyltransferase carries out the first enzymatic step of ''de novo''sphingolipid
Sphingolipids are a class of lipids containing a backbone of sphingoid bases, which are a set of aliphatic amino alcohols that includes sphingosine. They were discovered in brain extracts in the 1870s and were named after the mythological sp ...
biosynthesis
Biosynthesis, i.e., chemical synthesis occurring in biological contexts, is a term most often referring to multi-step, enzyme-Catalysis, catalyzed processes where chemical substances absorbed as nutrients (or previously converted through biosynthe ...
. The enzyme catalyses a Claisen-like condensation between L-serine
Serine
(symbol Ser or S) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α- amino group (which is in the protonated − form under biological conditions), a carboxyl group (which is in the deprotonated − ...
and an acyl-CoA thioester
In organic chemistry, thioesters are organosulfur compounds with the molecular structure . They are analogous to carboxylate esters () with the sulfur in the thioester replacing oxygen in the carboxylate ester, as implied by the thio- prefix ...
(CoASH) substrate (typically C16-palmitoyl) or an acyl-ACP (acyl-carrier protein) thioester substrate, to form 3-ketodihydrosphingosine. Initially PLP cofactor is bound to the active-site lysine via a Schiff base
In organic chemistry, a Schiff base (named after Hugo Schiff) is a compound with the general structure ( = alkyl or aryl, but not hydrogen). They can be considered a sub-class of imines, being either secondary ketimines or secondary aldim ...
to form the holo-form or internal aldimine of the enzyme. The amine
In chemistry, amines (, ) are organic compounds that contain carbon-nitrogen bonds. Amines are formed when one or more hydrogen atoms in ammonia are replaced by alkyl or aryl groups. The nitrogen atom in an amine possesses a lone pair of elec ...
group of L-serine then attacks and displaces the lysine bound to PLP, forming the external aldimine intermediate. Subsequently, deprotonation
Deprotonation (or dehydronation) is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction.Henry Jakubowski, Biochemistry Online Chapter 2A3, https://employees.csbsju.ed ...
occurs at the Cα of serine, forming the quinonoid intermediate that attacks the incoming thioester substrate. Following decarboxylation
Decarboxylation is a chemical reaction that removes a carboxyl group and releases carbon dioxide (CO2). Usually, decarboxylation refers to a reaction of carboxylic acids, removing a carbon atom from a carbon chain. The reverse process, which is ...
and lysine attack, the product 3-keto-dihydrosphingosine is released and catalytically active PLP is reformed. This condensation reaction forms the sphingoid base or long-chain base found in all subsequent intermediate sphingolipids and complex sphingolipids in the organism.
Isoforms
A variety of different serine ''C''-palmitoyltransferaseisoforms
A protein isoform, or "protein variant", is a member of a set of highly similar proteins that originate from a single gene and are the result of genetic differences. While many perform the same or similar biological roles, some isoforms have uniqu ...
exist across different species. Unlike in eukaryote
The eukaryotes ( ) constitute the Domain (biology), domain of Eukaryota or Eukarya, organisms whose Cell (biology), cells have a membrane-bound cell nucleus, nucleus. All animals, plants, Fungus, fungi, seaweeds, and many unicellular organisms ...
s, where the enzyme is heterodimeric and membrane bound, bacterial enzymes are homodimers and cytoplasm
The cytoplasm describes all the material within a eukaryotic or prokaryotic cell, enclosed by the cell membrane, including the organelles and excluding the nucleus in eukaryotic cells. The material inside the nucleus of a eukaryotic cell a ...
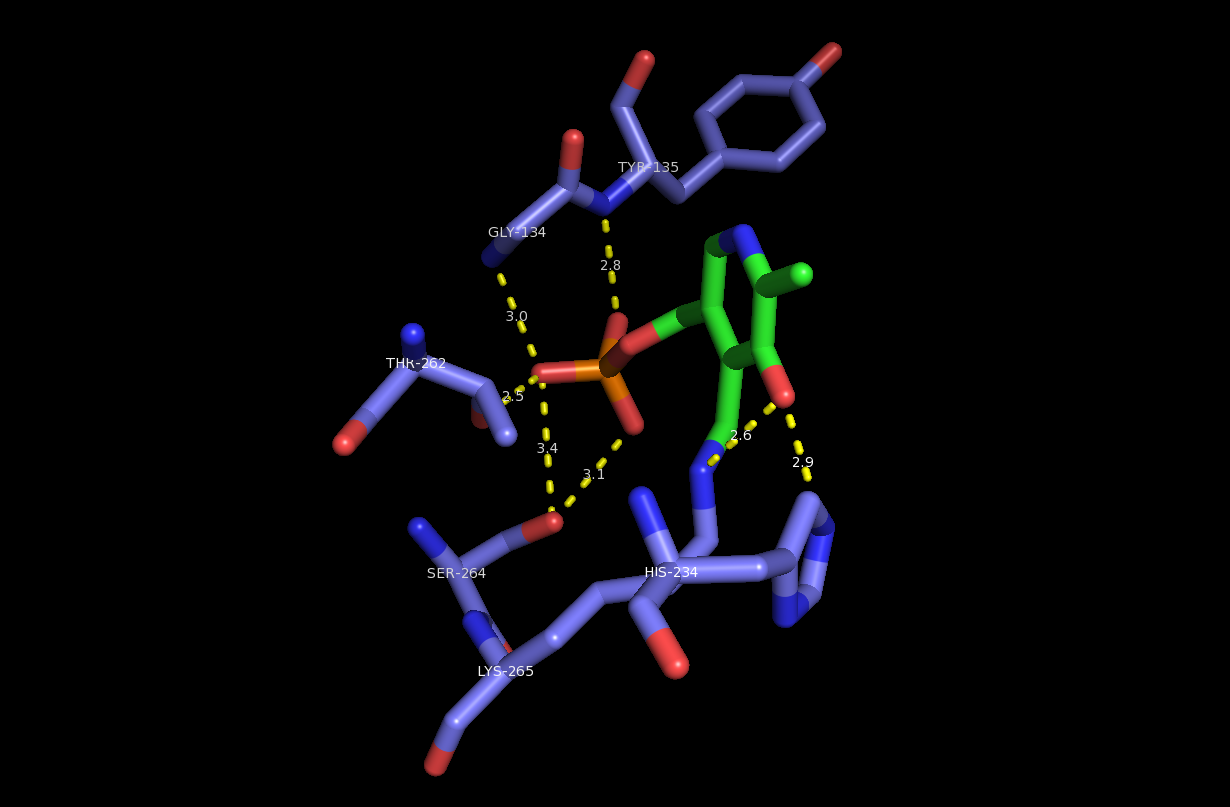
ic. Studies of the isoform of the enzyme found in the Gram-negative bacterium '' Sphingomonas paucimobilis'' were the first to elucidate the structure of the enzyme, revealing that PLP cofactor is held in place by several active site
In biology and biochemistry, the active site is the region of an enzyme where substrate molecules bind and undergo a chemical reaction. The active site consists of amino acid residues that form temporary bonds with the substrate, the ''binding s ...
residues including Lys265 and His159. Specifically, the ''S. paucimobilis'' isoform features an active-site arginine
Arginine is the amino acid with the formula (H2N)(HN)CN(H)(CH2)3CH(NH2)CO2H. The molecule features a guanidinium, guanidino group appended to a standard amino acid framework. At physiological pH, the carboxylic acid is deprotonated (−CO2−) a ...
residue (Arg378) that plays a key role in stabilizing the carboxy moiety of the PLP-L-serine external aldimine intermediate. Similar arginine residues in enzyme homologues (Arg370, Arg390) play analogous roles.
Other homologues, such as in '' Sphingobacterium multivorum'', feature the carboxy moiety bound to serine and methionine
Methionine (symbol Met or M) () is an essential amino acid in humans.
As the precursor of other non-essential amino acids such as cysteine and taurine, versatile compounds such as SAM-e, and the important antioxidant glutathione, methionine play ...
residues via water in place of arginine. Certain enzyme homologues, such as in ''S. multivorum'' as well as '' Bdellovibrio stolpii'', are found to be associated with the inner cell membrane, thus resembling the eukaryotic enzymes. The ''B. stolpii'' homologue also features substrate inhibition by palmitoyl-CoA, a feature shared by the yeast
Yeasts are eukaryotic, single-celled microorganisms classified as members of the fungus kingdom (biology), kingdom. The first yeast originated hundreds of millions of years ago, and at least 1,500 species are currently recognized. They are est ...
and mammalian homologues.
Clinical significance
HSAN1 (hereditary sensory and autonomic neuropathy type 1) is agenetic disorder
A genetic disorder is a health problem caused by one or more abnormalities in the genome. It can be caused by a mutation in a single gene (monogenic) or multiple genes (polygenic) or by a chromosome abnormality. Although polygenic disorders ...
caused by mutation
In biology, a mutation is an alteration in the nucleic acid sequence of the genome of an organism, virus, or extrachromosomal DNA. Viral genomes contain either DNA or RNA. Mutations result from errors during DNA or viral replication, ...
s in either one of ''SPTLC1'' or ''SPTLC2'', genes encoding the two heterodimeric subunits of the eukaryotic serine C-palmitoyltransferase enzyme. These mutations have been shown to alter active site specificity, specifically by enhancing the ability of the enzyme to condense -alanine with the palmitoyl-CoA substrate. This is consistent with elevated levels of deoxysphingoid bases formed by the condensation of alanine with palmitoyl-CoA observed in HSAN1 patients.
Species distribution
Serine ''C''-palmitoyltransferase is expressed in a large number of species from bacteria to humans. The bacterial enzyme is a water-soluble homodimer whereas ineukaryote
The eukaryotes ( ) constitute the Domain (biology), domain of Eukaryota or Eukarya, organisms whose Cell (biology), cells have a membrane-bound cell nucleus, nucleus. All animals, plants, Fungus, fungi, seaweeds, and many unicellular organisms ...
s the enzyme is a heterodimer which is anchored to the endoplasmic reticulum
The endoplasmic reticulum (ER) is a part of a transportation system of the eukaryote, eukaryotic cell, and has many other important functions such as protein folding. The word endoplasmic means "within the cytoplasm", and reticulum is Latin for ...
. Humans and other mammals express three paralogous subunits SPTLC1, SPTLC2, and SPTLC3. It was originally proposed that the functional human enzyme is a heterodimer between a SPTLC1 subunit and a second subunit which is either SPTLC2 or SPTLC3. However more recent data suggest that the enzyme may exist as a larger complex, possibly an octamer, comprising all three subunits.
References
{{Portal bar, Biology, border=no EC 2.3.1 Pyridoxal phosphate enzymes Enzymes of known structure