drug
A drug is any chemical substance other than a nutrient or an essential dietary ingredient, which, when administered to a living organism, produces a biological effect. Consumption of drugs can be via insufflation (medicine), inhalation, drug i ...
s that act on estrogen receptor
Estrogen receptors (ERs) are proteins found in cell (biology), cells that function as receptor (biochemistry), receptors for the hormone estrogen (17β-estradiol). There are two main classes of ERs. The first includes the intracellular estrogen ...
s (ERs). Compared to pure ER agonist
An agonist is a chemical that activates a Receptor (biochemistry), receptor to produce a biological response. Receptors are Cell (biology), cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an R ...
s–antagonists
An antagonist is a character in a story who is presented as the main enemy or rival of the protagonist and is often depicted as a villain.full agonist
An agonist is a chemical that activates a receptor to produce a biological response. Receptors are cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an antagonist blocks the action of the agoni ...
s and silent antagonist
A receptor antagonist is a type of receptor ligand or drug that blocks or dampens a biological response by binding to and blocking a receptor rather than activating it like an agonist. Antagonist drugs interfere in the natural operation of recep ...
s), SERMs are more tissue-specific, allowing them to selectively inhibit or stimulate estrogen
Estrogen (also spelled oestrogen in British English; see spelling differences) is a category of sex hormone responsible for the development and regulation of the female reproductive system and secondary sex characteristics. There are three ...
-like action in various tissues.
Medical uses
SERMs are used for various estrogen-related diseases, including treatment of ovulatory dysfunction in the management ofinfertility
In biology, infertility is the inability of a male and female organism to Sexual reproduction, reproduce. It is usually not the natural state of a healthy organism that has reached sexual maturity, so children who have not undergone puberty, whi ...
treatment, prevention of postmenopausal osteoporosis, treatment and risk reduction of breast cancer
Breast cancer is a cancer that develops from breast tissue. Signs of breast cancer may include a Breast lump, lump in the breast, a change in breast shape, dimpling of the skin, Milk-rejection sign, milk rejection, fluid coming from the nipp ...
, and treatment of dyspareunia
Dyspareunia ( ) is painful sexual intercourse due to somatic or psychological causes. The term ''dyspareunia'' covers both female dyspareunia and male dyspareunia, but many discussions that use the term without further specification concern the f ...
due to menopause
Menopause, also known as the climacteric, is the time when Menstruation, menstrual periods permanently stop, marking the end of the Human reproduction, reproductive stage for the female human. It typically occurs between the ages of 45 and 5 ...
. SERMs are also used in combination with conjugated estrogens
Conjugated estrogens (CEs), or conjugated equine estrogens (CEEs), sold under the brand name Premarin among others, is an estrogen medication which is used in menopausal hormone therapy and for various other indications. It is a mixture of th ...
indicated for the management of estrogen deficiency symptoms and of vasomotor
Vasomotor refers to actions upon a blood vessel which alter its diameter. More specifically, it can refer to vasodilator action and vasoconstrictor action.
Control Sympathetic innervation
Sympathetic nerve fibers travel around the tunica media ...
symptoms associated with menopause.
SERMs are also being explored for gender-affirming hormone therapy
Gender-affirming hormone therapy (GAHT), also called hormone replacement therapy (HRT) or transgender hormone therapy, is a form of hormone therapy in which sex hormones and other sex-hormonal agent, hormonal medications are administered to transg ...
in some non-binary
Non-binary or genderqueer Gender identity, gender identities are those that are outside the male/female gender binary. Non-binary identities often fall under the transgender umbrella since non-binary people typically identify with a gende ...
transgender individuals that were assigned male at birth. Unlike full estrogen receptor agonists like estradiol
Estradiol (E2), also called oestrogen, oestradiol, is an estrogen steroid hormone and the major female sex hormone. It is involved in the regulation of female reproductive cycles such as estrous and menstrual cycles. Estradiol is responsible ...
which cause the broad development of feminine secondary sex characteristics
A secondary sex characteristic is a physical characteristic of an organism that is related to or derived from its sex, but not directly part of its reproductive system. In humans, these characteristics typically start to appear during puberty ...
, SERMs can be used to achieve partial feminization in individuals who wish to develop certain feminine traits such as softer skin and feminine body fat distribution without significant breast growth. Unlike bioidentical estrogens, SERMs themselves do not suppress testosterone production and therefore are used alongside antiandrogens
Antiandrogens, also known as androgen antagonists or testosterone blockers, are a class of drugs that prevent androgens like testosterone and dihydrotestosterone (DHT) from mediating their biological effects in the body. They act by blocking th ...
such as cyproterone acetate
Cyproterone acetate (CPA), sold alone under the brand name Androcur or Ethinylestradiol/cyproterone acetate, with ethinylestradiol under the brand names Diane or Diane-35 among others, is an antiandrogen and progestin medication used in the tre ...
or spironolactone
Spironolactone, sold under the brand name Aldactone among others, is classed as a diuretic medication. It can be used to treat edema, fluid build-up due to hepatic cirrhosis, liver disease or kidney disease. It is also used to reduce risk o ...
. The use of SERMs for gender-affirming hormone therapy is still relatively new and uncommon as there is limited research into their efficacy and safety when used long-term.
Examples
Tamoxifen
Tamoxifen, sold under the brand name Nolvadex among others, is a selective estrogen receptor modulator used to prevent breast cancer in women and men. It is also being studied for other types of cancer. It has been used for Albright syndrome ...
is a first-line hormonal treatment for ER-positive metastatic breast cancer
Breast cancer is a cancer that develops from breast tissue. Signs of breast cancer may include a Breast lump, lump in the breast, a change in breast shape, dimpling of the skin, Milk-rejection sign, milk rejection, fluid coming from the nipp ...
. It is used for breast cancer risk reduction in women at high risk, and as adjuvant treatment for axillary node-negative and node-positive ductal carcinoma ''in situ
is a Latin phrase meaning 'in place' or 'on site', derived from ' ('in') and ' ( ablative of ''situs'', ). The term typically refers to the examination or occurrence of a process within its original context, without relocation. The term is use ...
''. Tamoxifen treatment is also used for the treatment of osteoporosis
Osteoporosis is a systemic skeletal disorder characterized by low bone mass, micro-architectural deterioration of bone tissue leading to more porous bone, and consequent increase in Bone fracture, fracture risk.
It is the most common reason f ...
and blood lipids in postmenopausal women. Adverse effects of tamoxifen include hot flash
Hot flushes are a form of flushing, often caused by the changing hormone levels that are characteristic of menopause. They are typically experienced as a feeling of intense heat with sweating and rapid heartbeat, and may typically last from t ...
es and an increase in the risk of developing endometrial cancer
Endometrial cancer is a cancer that arises from the endometrium (the epithelium, lining of the uterus or womb). It is the result of the abnormal growth of cells (biology), cells that can invade or spread to other parts of the body. The first s ...
compared to women of similar age.
Toremifene, a chlorinated
In chemistry, halogenation is a chemical reaction which introduces one or more halogens into a chemical compound. Halide-containing compounds are pervasive, making this type of transformation important, e.g. in the production of polymers, drugs. ...
tamoxifen derivative developed to avoid hepatic carcinomas, was associated with fewer DNA adducts in the liver than tamoxifen in preclinical studies. It is used as endocrine
The endocrine system is a messenger system in an organism comprising feedback loops of hormones that are released by internal glands directly into the circulatory system and that target and regulate distant organs. In vertebrates, the hypotha ...
therapy for women with estrogen or progesterone receptor
The progesterone receptor (PR), also known as NR3C3 or nuclear receptor subfamily 3, group C, member 3, is a protein found inside cells. It is activated by the steroid hormone progesterone.
In humans, PR is encoded by a single ''PGR'' gene resi ...
-positive, stage 4 or recurrent metastatic breast cancer and has demonstrated similar efficacy compared to tamoxifen as adjuvant treatment of breast cancer and in the treatment of metastatic breast cancer.
Raloxifene is used for the prevention and treatment of postmenopausal
Menopause, also known as the climacteric, is the time when menstrual periods permanently stop, marking the end of the reproductive stage for the female human. It typically occurs between the ages of 45 and 55, although the exact timing can ...
osteoporosis and breast cancer prevention in high-risk postmenopausal women with osteoporosis. Preclinical and clinical reports suggest that it is considerably less potent than estrogen for the treatment of osteoporosis. It is associated with an acceptable endometrial profile and has not demonstrated tamoxifen-like effects on the uterus, but has been associated with adverse effects such as venous thromboembolism
Venous thrombosis is the blockage of a vein caused by a thrombus (blood clot). A common form of venous thrombosis is deep vein thrombosis (DVT), when a blood clot forms in the deep veins. If a thrombus breaks off ( embolizes) and flows to the lun ...
and vasomotor symptoms, including hot flushes.
Ospemifene is an analogous metabolite
In biochemistry, a metabolite is an intermediate or end product of metabolism.
The term is usually used for small molecules. Metabolites have various functions, including fuel, structure, signaling, stimulatory and inhibitory effects on enzymes, c ...
of toremifene. Unlike tamoxifen, toremifene is not a rat hepatocarcinogen, and therefore ospemifene would also be a safer SERM than tamoxifen. It is used for the treatment of moderate to severe dyspareunia, a symptom of vulva
In mammals, the vulva (: vulvas or vulvae) comprises mostly external, visible structures of the female sex organ, genitalia leading into the interior of the female reproductive tract. For humans, it includes the mons pubis, labia majora, lab ...
r and vaginal atrophy associated with menopause. Clinical data on breast cancer are not available, but both ''in vitro'' and ''in vivo'' data suggest that ospemifene may have chemopreventive
Chemotherapy (often abbreviated chemo, sometimes CTX and CTx) is the type of cancer treatment that uses one or more anti-cancer drugs ( chemotherapeutic agents or alkylating agents) in a standard regimen. Chemotherapy may be given with a cu ...
activity in breast tissue.
Bazedoxifene is used for treatment of osteoporosis in postmenopausal women at increased risk of fracture
Fracture is the appearance of a crack or complete separation of an object or material into two or more pieces under the action of stress (mechanics), stress. The fracture of a solid usually occurs due to the development of certain displacemen ...
. It has been shown to be relatively safe and well-tolerated. It shows no breast or endometrial stimulation and in the first two years the small increase is better in venous thromboembolism, and similar in the long term to other SERMs. The advantage of bazedoxifene over raloxifene is that it increases endothelial nitric oxide synthase activity and does not antagonize the effect of 17β-estradiol on vasomotor symptoms.
The first tissue-selective estrogen complex (TSEC) combines conjugated estrogens
Conjugated estrogens (CEs), or conjugated equine estrogens (CEEs), sold under the brand name Premarin among others, is an estrogen medication which is used in menopausal hormone therapy and for various other indications. It is a mixture of th ...
and the SERM bazedoxifene to blend their activities. The combination therapy is used in the treatment of moderate to severe vasomotor symptoms associated with menopause, prevention of postmenopausal osteoporosis as well as treatment of estrogen deficiency symptoms in non- hysterectomized postmenopausal women. The combination allows for the benefits of estrogen with regard to relief of vasomotor symptoms without estrogenic stimulation of the endometrium
The endometrium is the inner epithelium, epithelial layer, along with its mucous membrane, of the mammalian uterus. It has a basal layer and a functional layer: the basal layer contains stem cells which regenerate the functional layer. The funct ...
.
SERMs have also been used in hormone replacement therapy
Hormone replacement therapy (HRT), also known as menopausal hormone therapy or postmenopausal hormone therapy, is a form of hormone therapy used to treat symptoms associated with female menopause. Effects of menopause can include symptoms such ...
by some transgender
A transgender (often shortened to trans) person has a gender identity different from that typically associated with the sex they were sex assignment, assigned at birth.
The opposite of ''transgender'' is ''cisgender'', which describes perso ...
people.
DHED is a centrally selective, orally active prodrug of estradiol.
Available forms
Pharmacology
Pharmacodynamics
SERMs are competitive partial agonists of the ER. Different tissues have different degrees of sensitivity to the activity of endogenous estrogens, so SERMs produce estrogenic orantiestrogen
Antiestrogens, also known as estrogen antagonists or estrogen blockers, are a class of drugs which prevent estrogens like estradiol from mediating their biological effects in the body. They act by blocking the estrogen receptor (ER) and/or inh ...
ic effects depending on the tissue in question, as well as the percentage of intrinsic activity
Intrinsic activity (IA) and efficacy (Emax) refer to the relative ability of a drug- receptor complex to produce a maximum functional response. This must be distinguished from the affinity, which is a measure of the ability of the drug to bind ...
(IA) of the SERM. An example of a SERM with high IA and thus mostly estrogenic effects is chlorotrianisene
Chlorotrianisene (CTA), also known as tri-''p''-anisylchloroethylene (TACE) and sold under the brand name Tace among others, is a nonsteroidal estrogen related to diethylstilbestrol (DES) which was previously used in the treatment of menopausal ...
, while an example of a SERM with low IA and thus mostly antiestrogenic effects is ethamoxytriphetol. SERMs like clomifene
Clomifene, also known as clomiphene, is a medication used to treat infertility in women who do not ovulate, including those with polycystic ovary syndrome. It is taken by mouth.
Common side effects include pelvic pain and hot flashes. Oth ...
and tamoxifen
Tamoxifen, sold under the brand name Nolvadex among others, is a selective estrogen receptor modulator used to prevent breast cancer in women and men. It is also being studied for other types of cancer. It has been used for Albright syndrome ...
are comparatively more in the middle in their IA and their balance of estrogenic and antiestrogenic activity. Raloxifene is a SERM that is more antiestrogenic than tamoxifen; both are estrogenic in bone, but raloxifene is antiestrogenic in the uterus
The uterus (from Latin ''uterus'', : uteri or uteruses) or womb () is the hollow organ, organ in the reproductive system of most female mammals, including humans, that accommodates the embryonic development, embryonic and prenatal development, f ...
while tamoxifen is estrogenic in this part of the body.
Binding site
SERM act on the estrogen receptor (ER), which is anintracellular
This glossary of biology terms is a list of definitions of fundamental terms and concepts used in biology, the study of life and of living organisms. It is intended as introductory material for novices; for more specific and technical definitions ...
, ligand-dependent transcriptional activator
A transcriptional activator is a protein (transcription factor) that increases transcription of a gene or set of genes. Activators are considered to have ''positive'' control over gene expression, as they function to promote gene transcription and ...
and belongs to the nuclear receptor
In the field of molecular biology, nuclear receptors are a class of proteins responsible for sensing steroids, thyroid hormones, vitamins, and certain other molecules. These intracellular receptors work with other proteins to regulate the ex ...
family. Two different subtypes of ER have been identified, ERα
Estrogen receptor alpha (ERα), also known as NR3A1 (nuclear receptor subfamily 3, group A, member 1), is one of two main types of estrogen receptor, a nuclear receptor (mainly found as a chromatin-binding protein)
that is activated by the se ...
and ERβ
Estrogen receptor beta (ERβ) also known as NR3A2 (nuclear receptor subfamily 3, group A, member 2) is one of two main types of estrogen receptor—a nuclear receptor which is activated by the sex hormone estrogen. In humans ERβ is encoded by t ...
. ERα is considered the main medium where estrogen signals are transduced at the transcriptional level and is the predominant ER in the female reproductive tract and mammary glands while ERβ is primarily in vascular endothelial cells
The endothelium (: endothelia) is a single layer of squamous endothelial cells that line the interior surface of blood vessels and lymphatic vessels. The endothelium forms an interface between circulating blood or lymph in the lumen and the res ...
, bone, and male prostate tissue. ERα and ERβ concentration are known to be different in tissues during development, aging or disease state. Many characteristics are similar between these two types such as size (~600 and 530 amino acid
Amino acids are organic compounds that contain both amino and carboxylic acid functional groups. Although over 500 amino acids exist in nature, by far the most important are the 22 α-amino acids incorporated into proteins. Only these 22 a ...
s) and structure. ERα and ERβ share approximately 97% of the amino-acid sequence identity in the DNA-binding domain
A DNA-binding domain (DBD) is an independently folded protein domain that contains at least one structural motif that recognizes double- or single-stranded DNA. A DBD can recognize a specific DNA sequence (a recognition sequence) or have a gener ...
and about 56% in the ligand-binding domain. The main difference of the ligand-binding domains is determined by Leu-384 and Met-421 in ERα, which are replaced by Met-336 and Ile
Ile or ILE may refer to:
Ile
* Ile, a Puerto Rican singer
* Ile District (disambiguation), multiple places
* Ilé-Ifẹ̀, an ancient Yoruba city in south-western Nigeria
* Interlingue (ISO 639:ile), a planned language
* Isoleucine, an amino a ...
-373, respectively, in ERβ. The variation is greater on the N-terminus between ERα and ERβ.
DNA-binding domain consists of two subdomains. One with a proximal box that is involved in DNA recognition while the other contains a distal box responsible for DNA-dependent, DNA-binding domain dimerization
In chemistry, dimerization is the process of joining two identical or similar molecular entities by bonds. The resulting bonds can be either strong or weak. Many symmetrical chemical species are described as dimers, even when the monomer is u ...
. The proximal box sequence is identical between ERα and ERβ, which indicates similar specificity and affinity between the two subgroups. DNA-binding domain's globular proteins contain eight cysteine
Cysteine (; symbol Cys or C) is a semiessential proteinogenic amino acid with the chemical formula, formula . The thiol side chain in cysteine enables the formation of Disulfide, disulfide bonds, and often participates in enzymatic reactions as ...
s and allow for a tetrahedral coordination of two zinc
Zinc is a chemical element; it has symbol Zn and atomic number 30. It is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed. It is the first element in group 12 (IIB) of the periodic tabl ...
ions. This coordination makes the binding of ER to estrogen response elements possible. The ligand-binding domain is a globular, three-layered structure made of 11 helix
A helix (; ) is a shape like a cylindrical coil spring or the thread of a machine screw. It is a type of smooth space curve with tangent lines at a constant angle to a fixed axis. Helices are important in biology, as the DNA molecule is for ...
es and contains a pocket for the natural or synthetic ligand. Influencing factors for binding affinity are mainly the presence of a phenol
Phenol (also known as carbolic acid, phenolic acid, or benzenol) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile and can catch fire.
The molecule consists of a phenyl group () ...
moiety, molecular size and shape, double bonds and hydrophobicity
In chemistry, hydrophobicity is the chemical property of a molecule (called a hydrophobe) that is seemingly intermolecular force, repelled from a mass of water. In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to b ...
.
The differential positioning of the activating function 2 (AF-2) helix 12 in the ligand-binding domain by the bound ligand determines whether the ligand has an agonistic and antagonistic effect. In agonist-bound receptors, helix 12 is positioned adjacent to helices 3 and 5. Helices 3, 5, and 12 together form a binding surface for an NR box motif contained in coactivators with the canonical sequence LXXLL (where L represents leucine
Leucine (symbol Leu or L) is an essential amino acid that is used in the biosynthesis of proteins. Leucine is an α-amino acid, meaning it contains an α-amino group (which is in the protonated −NH3+ form under biological conditions), an α-Car ...
or isoleucine
Isoleucine (symbol Ile or I) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH form under biological conditions), an α-carboxylic acid group (which is in the depro ...
and X is any amino acid). Unliganded (apo) receptors or receptors bound to antagonist ligands turn helix 12 away from the LXXLL-binding surface that leads to preferential binding of a longer leucine-rich motif, LXXXIXXX(I/L), present on the corepressor
In genetics and molecular biology, a corepressor is a molecule that represses the expression of genes. In prokaryotes
A prokaryote (; less commonly spelled procaryote) is a single-celled organism whose cell lacks a nucleus and other membra ...
s NCoR1 or SMRT. In addition, some cofactors bind to ER through the terminals, the DNA-binding site or other binding sites. Thus, one compound can be an ER agonist in a tissue rich in coactivators but an ER antagonist in tissues rich in corepressors.
Mechanism of action
Estrogenic compounds span a spectrum of activity, including: * Full agonists (agonistic in all tissues), such as the natural endogenous hormoneestradiol
Estradiol (E2), also called oestrogen, oestradiol, is an estrogen steroid hormone and the major female sex hormone. It is involved in the regulation of female reproductive cycles such as estrous and menstrual cycles. Estradiol is responsible ...
.
* Mixed agonists/antagonistic (agonistic in some tissues while antagonistic in others), such as tamoxifen (a SERM).
* Pure antagonists (antagonistic in all tissues), such as fulvestrant.
SERMs are known to stimulate estrogenic actions in tissues such as the liver, bone and cardiovascular system but known to block estrogen action where stimulation is not desirable, such as in the breast and the uterus. This agonistic or antagonistic activity causes varied structural changes of the receptors, which results in activation or repression of the estrogen target genes. SERMs interact with receptors by diffusing into cells and their binding to ERα or ERβ subunits, which results in dimerization
In chemistry, dimerization is the process of joining two identical or similar molecular entities by bonds. The resulting bonds can be either strong or weak. Many symmetrical chemical species are described as dimers, even when the monomer is u ...
and structural changes of the receptors. This makes it easier for the SERMs to interact with estrogen response elements which leads to the activation of estrogen-inducible genes and mediating the estrogen effects.
SERMs unique feature is their tissue- and cell-selective activity. There is growing evidence to support that SERM activity is mainly determined by selective recruitment of corepressors and coactivators to ER target genes in specific types of tissues and cells. SERMs can impact coactivator protein stability and can also regulate coactivator activity through post-translational modification
In molecular biology, post-translational modification (PTM) is the covalent process of changing proteins following protein biosynthesis. PTMs may involve enzymes or occur spontaneously. Proteins are created by ribosomes, which translation (biolog ...
s such as phosphorylation
In biochemistry, phosphorylation is described as the "transfer of a phosphate group" from a donor to an acceptor. A common phosphorylating agent (phosphate donor) is ATP and a common family of acceptor are alcohols:
:
This equation can be writ ...
. Multiple growth signaling pathways, such as HER2
Receptor tyrosine-protein kinase erbB-2 is a protein that normally resides in the membranes of cells and is encoded by the ''ERBB2'' gene. ERBB is abbreviated from erythroblastic oncogene B, a gene originally isolated from the avian genome. The ...
, PKC, PI3K
Phosphoinositide 3-kinases (PI3Ks), also called phosphatidylinositol 3-kinases, are a family of enzymes involved in cellular functions such as cell growth, proliferation, differentiation, motility, survival and intracellular trafficking, which i ...
and more, are downregulated in response to anti-estrogen treatment. Steroid receptor coactivator 3 (SRC-3) is phosphorylated by activated kinase
In biochemistry, a kinase () is an enzyme that catalyzes the transfer of phosphate groups from high-energy, phosphate-donating molecules to specific substrates. This process is known as phosphorylation, where the high-energy ATP molecule don ...
s that also enhance its coactivator activity, affect cell growth and ultimately contribute to drug resistance.
The ratio of ERα and ERβ at a target site may be another way SERM activity is determined. High levels of cellular proliferation correlate well with a high ERα:ERβ ratio, but repression of cellular proliferation correlates to ERβ being dominant over ERα. The ratio of ERs in neoplastic
A neoplasm () is a type of abnormal and excessive growth of tissue. The process that occurs to form or produce a neoplasm is called neoplasia. The growth of a neoplasm is uncoordinated with that of the normal surrounding tissue, and persists ...
and normal breast tissue could be important when considering chemoprevention with SERMs.
When looking at the differences between ERα and ERβ, Activating Function 1 (AF-1) and AF-2 are important. Together they play an important part in the interaction with other co-regulatory proteins that control gene transcription
Transcription is the process of copying a segment of DNA into RNA for the purpose of gene expression. Some segments of DNA are transcribed into RNA molecules that can encode proteins, called messenger RNA (mRNA). Other segments of DNA are transc ...
. AF-1 is located in the amino terminus
The N-terminus (also known as the amino-terminus, NH2-terminus, N-terminal end or amine-terminus) is the start of a protein or polypeptide, referring to the free amine group (-NH2) located at the end of a polypeptide. Within a peptide, the amin ...
of the ER and is only 20% homologous in ERα and ERβ. On the other hand, AF-2 is very similar in ERα and ERβ, and only one amino acid is different. Studies have shown that by switching AF-1 regions in ERα and ERβ, that there are specific differences in transcription activity. Generally, SERMs can partially activate engineered genes through ERα by an estrogen receptor element, but not through ERβ. Although, raloxifene and the active form of tamoxifen can stimulate AF-1-regulated reporter genes in both ERα and ERβ.
Because of the discovery that there are two ER subtypes, it has brought about the synthesis of a range of receptor specific ligands that can switch on or off a particular receptor. However, the external shape of the resulting complex is what becomes the catalyst for changing the response at a tissue target to a SERM.
X-ray crystallography
X-ray crystallography is the experimental science of determining the atomic and molecular structure of a crystal, in which the crystalline structure causes a beam of incident X-rays to Diffraction, diffract in specific directions. By measuring th ...
of estrogens or antiestrogens has shown how ligands program the receptor complex to interact with other proteins. The ligand-binding domain of the ER demonstrates how ligands promote and prevent coactivator binding based on the shape of the estrogen or antiestrogen complex. The broad range of ligands that bind to the ER can create a spectrum of ER complexes that are fully estrogenic or antiestrogenic at a specific target site. The main result of a ligand-binding to ER is a structural rearrangement of the ligand- binding pocket, primarily in the AF-2 of the C-terminal region. The binding of ligands to ER leads to the formation of a hydrophobic
In chemistry, hydrophobicity is the chemical property of a molecule (called a hydrophobe) that is seemingly repelled from a mass of water. In contrast, hydrophiles are attracted to water.
Hydrophobic molecules tend to be nonpolar and, thu ...
pocket that regulates cofactors and receptor pharmacology. The correct folding of ligand-binding domain is required for activation of transcription and for ER to interact with a number of coactivators.
Coactivators are not just protein partners that connect sites together in a complex. Coactivators play an active role in modifying the activity of a complex. Post-translation modification of coactivators can result in a dynamic model of steroid hormone
A steroid hormone is a steroid that acts as a hormone. Steroid hormones can be grouped into two classes: corticosteroids (typically made in the adrenal cortex, hence ''cortico-'') and sex steroids (typically made in the gonads or placenta). Wit ...
action by way of multiple kinase pathways initiated by cell surface growth factor receptor
A growth factor receptor is a receptor that binds to a growth factor. Growth factor receptors are the first stop in cells where the signaling cascade for cell differentiation and proliferation begins. Growth factors, which are ligands that bind to ...
s. Under the guidance of a multitude of protein remodelers to form a multiprotein coactivator complex that can interact with the phosphorylated ER at a specific gene promoter site, the core coactivator first has to recruit a specific set of cocoactivators. The proteins that the core coactivator assembles as the core coactivated complex have individual enzymatic activities to methylate or acetylate adjacent proteins. The ER substrates or coenzyme A
Coenzyme A (CoA, SHCoA, CoASH) is a coenzyme, notable for its role in the Fatty acid metabolism#Synthesis, synthesis and Fatty acid metabolism#.CE.B2-Oxidation, oxidation of fatty acids, and the oxidation of pyruvic acid, pyruvate in the citric ac ...
can be polyubiquitinated by multiple cycles of the reaction or, depending on linkage proteins, they can either be activated further or degraded by the 26S proteasome.
Consequently, to have an effective gene transcription that is programmed and targeted by the structure and phosphorylation status of the ER and coactivators, it is required to have a dynamic and cyclic process of remodeling capacity for transcriptional assembly, after which the transcription complex is then instantly routinely destroyed by the proteasome.
Structure and function
Structure–activity relationships
The core structure of SERMs simulates the 17β-estradiol template. They have two aromatic rings separated by 1-3 atoms (often astilbene Stilbene may refer to one of the two stereoisomers of 1,2-diphenylethene:
* (''E'')-Stilbene (''trans'' isomer)
* (''Z'')-Stilbene (''cis'' isomer)
See also
* Stilbenoid
Stilbenoids are hydroxylated derivatives of stilbene. They have a C6–C ...
-type of arrangement). Between the two phenyl
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula , and is often represented by the symbol Ph (archaically φ) or Ø. The phenyl group is closely related to benzene and can be viewed as a benzene ...
s of the core, SERMs typically have a 4-substituted phenyl group that, when bound to ER, projects from a position of an estratriene
Estrin (American English), or oestrin (British English), also known as estra-1,3,5(10)-triene, is an estrane steroid. It is dehydrogenated estrane with double bonds specifically at the C1, C3, and C5(10) positions. Estrin is a parent structure o ...
nucleus so that helix 12 moves from the receptor opening and blocks the space where coactivator proteins would normally bind and cause ER agonist activity. There has been a lot of variations in the core portion of SERMs while there has been less flexibility with what is tolerated in the side chain
In organic chemistry and biochemistry, a side chain is a substituent, chemical group that is attached to a core part of the molecule called the "main chain" or backbone chain, backbone. The side chain is a hydrocarbon branching element of a mo ...
. SERMs can be classified by their core structure.
First-generation triphenylethylenes
 The first main structural class of SERM-type molecules reported are the
The first main structural class of SERM-type molecules reported are the triphenylethylene
Triphenylethylene (TPE) is the organic compound with the formula . It is a colorless solid. Synthesis and reactions
The compound is prepared in two steps from benzophenone via the intermediacy of 1,2,2-triphenylethanol. Triphenylethylene reacts ...
s. The stilbene core (similar to the nonsteroidal estrogen, diethylstilbestrol) essentially mimics steroidal estrogens such as 17β-estradiol, while the side chain overlays with the 11th position of the steroid nucleus. Triphenylethylene derivatives have an additional phenyl group attached to the ethylene
Ethylene (IUPAC name: ethene) is a hydrocarbon which has the formula or . It is a colourless, flammable gas with a faint "sweet and musky" odour when pure. It is the simplest alkene (a hydrocarbon with carbon–carbon bond, carbon–carbon doub ...
bridge group. The 3-position H-bonding ability of phenols is a significant requirement for ER binding.
 The first drug, clomifene, has a chloro-
The first drug, clomifene, has a chloro-substituent
In organic chemistry, a substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule.
The suffix ''-yl'' is used when naming organic compounds that contain a single bond r ...
on the ethylene side chain which produces similar binding affinities as the later discovered drug tamoxifen. Clomifene is a mixture of estrogenic ( cis-form) and antiestrogenic isomer
In chemistry, isomers are molecules or polyatomic ions with identical molecular formula – that is, the same number of atoms of each element (chemistry), element – but distinct arrangements of atoms in space. ''Isomerism'' refers to the exi ...
s ( trans-form). Cis and trans are defined in terms of the geometric relationships of the two unsubstituted phenyl rings. The two isomers of clomifene have different profiles, where the trans-form has activity more similar to tamoxifen while the cis-form behaves more like 17β-estradiol. Cis is approximately ten times more potent than trans. However, trans isomer is the most potent stimulator of epithelial cell hypertrophy since clomifene is antagonistic at low doses and agonistic at high doses. The antagonist isomers may cause inhibitory estrogenic effects in the uterus and mammary cancers, but the estrogenic isomer could combine with novel receptors to produce estrogen-like effects in bone.
alkylation Alkylation is a chemical reaction that entails transfer of an alkyl group. The alkyl group may be transferred as an alkyl carbocation, a free radical, a carbanion, or a carbene (or their equivalents). Alkylating agents are reagents for effecting al ...
and strand scission. This problem is later corrected in toremifene. Tamoxifen is more promiscuous than raloxifene in target sites because of the relationship between ER's amino acid in Asp-351 and the antiestrogenic side chain of the SERM. The side chain for tamoxifen cannot neutralize Asp-351, so the site allosterically influences AF-1 at the proximal end of the ER. This issue is mended with the second-generation drug raloxifene.
Toremifene is a chlorinated derivative of the nonsteroidal triphenylethylene antiestrogen tamoxifen with a chloro substituent at the ethylene side chain producing similar binding affinities to that of tamoxifen. The structure and activity relationship of toremifene is similar to that of tamoxifen, but it has a substantial improvement from the older drug in regards to DNA alkylation. The presence of the added chlorine atom reduces the stability of cation
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
s formed from activated allylic metabolites and thus decreases alkylation potential, and indeed toremifene does not display DNA adduct formation in rodent hepatocyte
A hepatocyte is a cell of the main parenchymal tissue of the liver. Hepatocytes make up 80% of the liver's mass.
These cells are involved in:
* Protein synthesis
* Protein storage
* Transformation of carbohydrates
* Synthesis of cholesterol, bi ...
s. Toremifene protects against bone loss in ovariectomized rat models and affects bone resorption markers clinically in a similar fashion to tamoxifen. Toremifene undergoes phase I metabolism by microsomal cytochrome P450 enzymes, like tamoxifen, but primarily by the CYP3A4 isoform. Toremifene forms its two major metabolites N-desmethyltoremifene and deaminohydroxy-toremifene (ospemifene) by undergoing N-demethylation and deamination-hydroxylation. N-desmethyltoremifene has similar efficacy as toremifene while 4-hydroxytoremifene has a higher binding affinity to the ER than toremifene. 4-hydroxytoremifene has a role similar to that of 4-hydroxytamoxifen.
Second-generation benzothiophenes
Raloxifene belongs to the second-generationbenzothiophene
Benzothiophene is an aromatic organic compound with a molecular formula C8H6S and an odor similar to naphthalene (mothballs). It occurs naturally as a constituent of petroleum-related deposits such as lignite tar. Benzothiophene has no househol ...
SERM drugs. It has a high affinity for the ER with potent antiestrogenic activity and tissue-specific effects distinct from estradiol. Raloxifene is an ER agonist in bone and the cardiovascular system, but in breast tissue and the endometrium it acts as an ER antagonist. It is extensively metabolized by glucuronide conjugation in the gut and because of that has a low bioavailability
In pharmacology, bioavailability is a subcategory of absorption and is the fraction (%) of an administered drug that reaches the systemic circulation.
By definition, when a medication is administered intravenously, its bioavailability is 100%. H ...
of only 2% while that of tamoxifen and toremifene is approximately 100%.
The advantage of raloxifene over the triphenylethylene tamoxifen is reduced effect on the uterus. The flexible hinge group, as well as the antiestrogenic phenyl 4-piperidinoethoxy side chain, are important for minimizing uterine effects. Because of its flexibility the side chain can obtain an orthogonal disposition relative to the core so that the amine of raloxifene side chain is 1 Å closer than tamoxifens to amino acid Asp-351 in ERα's ligand-binding domain.
The critical role of the intimate relationship between the hydrophobic side chain of raloxifene and the hydrophobic residue of the receptor to change both the shape and charge of the external surface of a SERM-ER complex has been confirmed with raloxifene derivatives. When the interactive distance between raloxifene and Asp-351 is increased from 2.7 Å to 3.5-5 Å it causes increased estrogen-like action of the raloxifene-ERα complex. When the piperidine ring of raloxifene is replaced by cyclohexane
Cyclohexane is a cycloalkane with the molecular formula . Cyclohexane is non-polar. Cyclohexane is a colourless, flammable liquid with a distinctive detergent-like odor, reminiscent of cleaning products (in which it is sometimes used). Cyclohexan ...
, the ligand loses antiestrogenic properties and becomes a full agonist. The interaction between SERM's antiestrogenic side chain and amino acid Asp-351 is the important first step in silencing AF-2. It relocates helix 12 away from the ligand-binding pocket thereby preventing coactivators from binding to the SERM-ER complex.
Third-generation
 Third-generation compounds display either no uterine stimulation, improved potency, no significant increases in hot flushes or a combination of these attributes.
The first dihydronapthalene SERM,
Third-generation compounds display either no uterine stimulation, improved potency, no significant increases in hot flushes or a combination of these attributes.
The first dihydronapthalene SERM, nafoxidine
Nafoxidine (; developmental code names U-11,000A) or nafoxidine hydrochloride () is a nonsteroidal selective estrogen receptor modulator (SERM) or partial agonist, partial antiestrogen of the triphenylethylene group that was developed for the tre ...
, was a clinical candidate for the treatment of breast cancer but had side effects including severe phototoxicity. Nafoxidine has all three phenyls constrained in a coplanar arrangement like tamoxifen. But with hydrogenation, the double bond of nafoxidene were reduced, and both phenyls are cis-oriented. The amine-bearing side chain can then adopt an axial conformation and locate this group orthogonally to the plane of the core, like ralofoxifene and other less uterotropic SERMs.
 Modifications of nafoxidine resulted in lasofoxifene. Lasofoxifene is among the most potent SERMs reported in protection against bone loss and cholesterol reduction. The excellent oral potency of lasofoxifene has been attributed to reduced intestinal glucuronidation of the phenol. Unlike raloxifene, lasofoxifene satisfies the requirement of a pharmacophore model that predicts resistance to gut wall glucuronidation. The structural requirement is a non-planar topology with the steric bulk close to the plane of a fused bicyclic aromatic system. The interactions between the ER and lasofoxifene are consistent with the general features of SERM-ER recognition. Lasofoxifene's large flexible side chain terminates in a pyrrolidine head group and threads its way out toward the surface of the protein, where it interferes directly with the positioning of the AF-2 helix. A salt bridge forms between lasofoxifene and Asp-351. The charge neutralization in this region ER may explain some antiestrogenic effects exerted by lasofoxifene.
Modifications of nafoxidine resulted in lasofoxifene. Lasofoxifene is among the most potent SERMs reported in protection against bone loss and cholesterol reduction. The excellent oral potency of lasofoxifene has been attributed to reduced intestinal glucuronidation of the phenol. Unlike raloxifene, lasofoxifene satisfies the requirement of a pharmacophore model that predicts resistance to gut wall glucuronidation. The structural requirement is a non-planar topology with the steric bulk close to the plane of a fused bicyclic aromatic system. The interactions between the ER and lasofoxifene are consistent with the general features of SERM-ER recognition. Lasofoxifene's large flexible side chain terminates in a pyrrolidine head group and threads its way out toward the surface of the protein, where it interferes directly with the positioning of the AF-2 helix. A salt bridge forms between lasofoxifene and Asp-351. The charge neutralization in this region ER may explain some antiestrogenic effects exerted by lasofoxifene.
 The indole system has served as a core unit in SERMs, and when an amine is attached to the indole with a benzyloxyethyl, the resultant compounds were shown to have no preclinical uterine activity while sparing rat bone with full efficacy at low doses. Bazedoxifene is one of those compounds. The core binding domain consists of a 2-phenyl-3-methyl indole and a hexamethylenamine ring at the side chain affecter region. It is metabolized by glucuronidation, with the absolute bioavailability of 6.2%, 3-fold higher than that of raloxifene. It has agonistic effects on bone and lipid metabolism but not on breast and uterine endometrium. It is well tolerated and displays no increase in hot flush , uterine hypertrophy or breast tenderness.
The indole system has served as a core unit in SERMs, and when an amine is attached to the indole with a benzyloxyethyl, the resultant compounds were shown to have no preclinical uterine activity while sparing rat bone with full efficacy at low doses. Bazedoxifene is one of those compounds. The core binding domain consists of a 2-phenyl-3-methyl indole and a hexamethylenamine ring at the side chain affecter region. It is metabolized by glucuronidation, with the absolute bioavailability of 6.2%, 3-fold higher than that of raloxifene. It has agonistic effects on bone and lipid metabolism but not on breast and uterine endometrium. It is well tolerated and displays no increase in hot flush , uterine hypertrophy or breast tenderness.
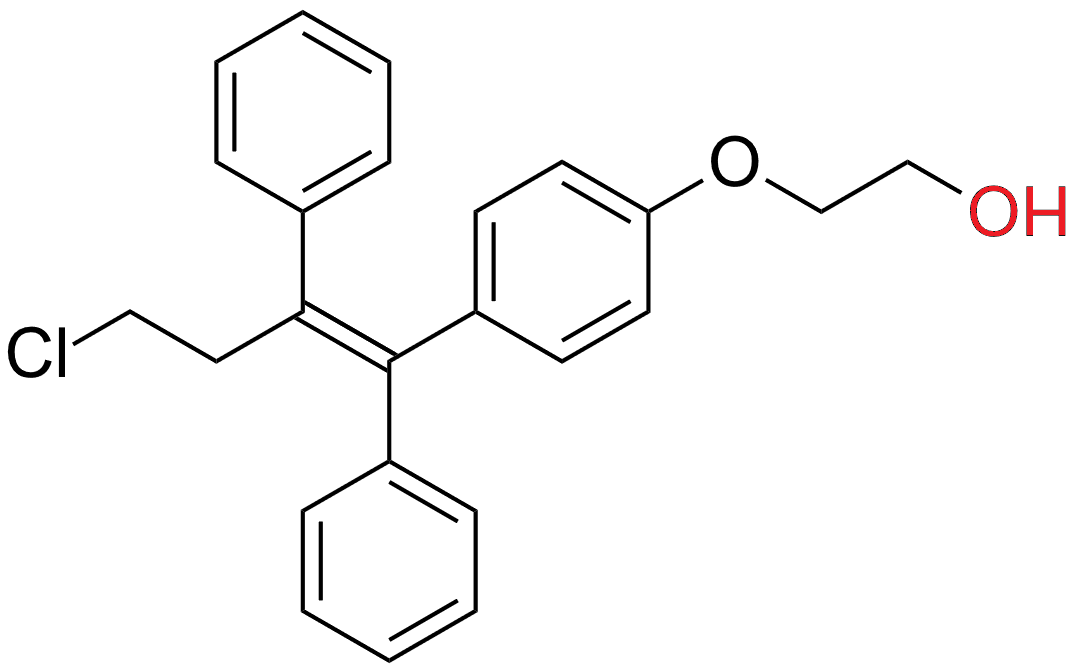 Ospemifene is a triphenylethylene and a known metabolite of toremifene. It's structurally very similar to tamoxifen and toremifene. Ospemifene does not have 2-(dimethylamino)ethoxy group as tamoxifen. Structure–activity relationship studies showed that by removing that group of tamoxifen agonistic activity in the uterus was significantly reduced, but not in bone and cardiovascular system. Preclinical and clinical data show that ospemifene is well tolerated with no major side effects. Benefits that ospemifene may have over other SERMs is its neutral effect on hot flushes and ER-agonist effect on the vagina, improving the symptoms of vaginal dryness.
Ospemifene is a triphenylethylene and a known metabolite of toremifene. It's structurally very similar to tamoxifen and toremifene. Ospemifene does not have 2-(dimethylamino)ethoxy group as tamoxifen. Structure–activity relationship studies showed that by removing that group of tamoxifen agonistic activity in the uterus was significantly reduced, but not in bone and cardiovascular system. Preclinical and clinical data show that ospemifene is well tolerated with no major side effects. Benefits that ospemifene may have over other SERMs is its neutral effect on hot flushes and ER-agonist effect on the vagina, improving the symptoms of vaginal dryness.
Binding modes
The SERMs are known to feature four distinctive modes of binding to ER. One of those features are stronghydrogen bonds
In chemistry, a hydrogen bond (H-bond) is a specific type of molecular interaction that exhibits partial covalent character and cannot be described as a purely electrostatic force. It occurs when a hydrogen (H) atom, covalently bonded to a mo ...
between the ligand and ERα's Arg-394 and Glu-353 that line the "A-ring pocket" and help the ligand to stay in ER's binding pocket. This is unlike 17β-estradiol which is hydrogen bonded to His-524 in the "D-ring pocket". Other distinctive bindings to the ligand-binding pocket are with a nearly planar "core" structure typically composed of a biaryl heterocycle
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic organic chemistry is the branch of organic chemistry dealing with the synthesis, proper ...
, equivalent to the A-ring and B-ring of 17β-estradiol, to the corresponding binding site; a bulky side chain from the biaryl structure, analogous to the B-ring of 17β-estradiol and finally a second side group that is the C- and D-ring equivalent and usually aromatic, fills the remainder volume of the ligand-binding pocket.
The small differences between the two subtypes of ER have been used to develop subtype-selective ER modulators, but the high similarity between the two receptors make the development very challenging. Amino acids in the ligand-binding domains differ at two positions, Leu-384 and Met-421 in ERα and Met-336 and Ile-373 in ERβ, but they have similar hydrophobicity and occupying volumes. However, the shapes and the rotational barrier of the amino acid residues are not the same, leading to distinguish α- and β-face of the binding cavity between ERα and ERβ. This causes ERα-preferential-binding of ligand substituent
In organic chemistry, a substituent is one or a group of atoms that replaces (one or more) atoms, thereby becoming a moiety in the resultant (new) molecule.
The suffix ''-yl'' is used when naming organic compounds that contain a single bond r ...
s that are aligned downwards facing Met-336 while ligand substituents aligned upwards facing Met-336 are more likely to bind to ERβ. Another difference is in Val-392 in ERα, which is replaced by Met-344 in ERβ. ERβ's binding pocket volume is slightly smaller and the shape a bit different from ERα's. Many ERβ-selective ligands have a largely planar arrangement as the binding cavity of ERβ is slightly narrower than that of ERα, however, this by itself leads to modest selectivity. To attain strong selectivity, the ligand must place substituents very close to one or more of the amino acid differences between ERα and ERβ in order to create a strong repulsive force towards the other subtype receptor. In addition, the structure of the ligand must be rigid. Repulsive interactions may otherwise lead to the conformational change of the ligand and, therefore, create alternative binding modes.
First-generation triphenylethylenes
Tamoxifen is converted by the livercytochrome P450
Cytochromes P450 (P450s or CYPs) are a Protein superfamily, superfamily of enzymes containing heme as a cofactor (biochemistry), cofactor that mostly, but not exclusively, function as monooxygenases. However, they are not omnipresent; for examp ...
into the 4-hydroxytamoxifen and is a more selective antagonist of the ERα subtype than ERβ. 4-hydroxytamoxifen binds to ERs within the same binding pocket that recognizes 17β-estradiol. The receptor recognition of 4-hydroxytamoxifen appears to be controlled by two structural features of 4-hydroxytamoxifen, the phenolic A ring, and the bulky side chain. The phenolic A ring forms hydrogen bonds to the side groups of ER's Arg-394, Glu-354 and to structurally conserved water. The bulky side chain, protruding from the binding cavity, displaces helix 12 from ligand-binding pocket to cover part of the coactivator binding pocket. The ER-4-hydroxytamoxifen complex formation recruits corepressors proteins. This leads to decreased DNA synthesis and inhibition of estrogen activity. Clomifene and torimefene produce binding affinities similar to that of tamoxifen. Thus, these two drugs are more selective antagonists of the ERα subtype than ERβ.
Second-generation benzothiophenes
 Raloxifene, like 4-hydroxytamoxifen, binds to ERα with the hydroxyl group of its phenolic "A ring" through hydrogen bonds with Arg-394 and Glu-353. In addition to these bonds, raloxifene forms a second hydrogen bond to ER through the side group of His-524 because of the presence of a second hydroxyl group in the "D ring". This hydrogen bond is also unlike that between 17β-estradiol and His-524, as the imidazole ring of His-524 is rotated to counteract the difference of the oxygen position in raloxifene and in 17β-estradiol. Just like in 4-hydroxytamoxifen, the bulky side chain of raloxifene displaces helix 12.
Raloxifene, like 4-hydroxytamoxifen, binds to ERα with the hydroxyl group of its phenolic "A ring" through hydrogen bonds with Arg-394 and Glu-353. In addition to these bonds, raloxifene forms a second hydrogen bond to ER through the side group of His-524 because of the presence of a second hydroxyl group in the "D ring". This hydrogen bond is also unlike that between 17β-estradiol and His-524, as the imidazole ring of His-524 is rotated to counteract the difference of the oxygen position in raloxifene and in 17β-estradiol. Just like in 4-hydroxytamoxifen, the bulky side chain of raloxifene displaces helix 12.
Third-generation
Lasofoxifene interaction with ERα is typical of those between SERM-ERα such as a nearly planartopology
Topology (from the Greek language, Greek words , and ) is the branch of mathematics concerned with the properties of a Mathematical object, geometric object that are preserved under Continuous function, continuous Deformation theory, deformat ...
(the tetrahydronapthalene carbocycle), hydrogen bonding with Arg-394 and Glu-353 and the phenyl side chains of lasofoxifene filling the C-ring and D-ring volume of the ligand-binding pocket. Lasofoxifene diverts helix 12 and prevents the binding of coactivator proteins with LXXLL motives. This is achieved by lasofoxifene occupying the space normally filled by Leu-540's side group and modulating the conformation of residues of helix 11 (His-524, Leu-525). Furthermore, lasofoxifene also directly interferes with helix 12 positioning by the drug's ethyl pyrrolidine group. In vitro studies indicate that bazedoxifene competitively blocks 17β-estradiol by high and similar binding to both ERα and ERβ. Bazedoxifenes main binding domain consists of the 2-phenyl-3-methylindole and a hexamethylenamine ring at the side chain affected region.
Ospemifene is an oxidative deaminated metabolite of toremifene as has a similar binding to ER as toremifene and tamoxifen. The competitive binding to ERα and ERβ of the three metabolites 4-hydroxy Ospemifene, 4'-hydroxy Ospemifene and the 4-hydroxy-, side chain carboxylic acid Ospemifene is at least as high as the parent compound.
History
Clomifene
Clomifene, also known as clomiphene, is a medication used to treat infertility in women who do not ovulate, including those with polycystic ovary syndrome. It is taken by mouth.
Common side effects include pelvic pain and hot flashes. Oth ...
and tamoxifen
Tamoxifen, sold under the brand name Nolvadex among others, is a selective estrogen receptor modulator used to prevent breast cancer in women and men. It is also being studied for other types of cancer. It has been used for Albright syndrome ...
prevented conception in rats but did the opposite in humans. Clomifene successfully induced ovulation in subfertile women and on February 1, 1967, it was approved in the US for the treatment of ovulation dysfunction in women who were trying to conceive. Toxicological
Toxicology is a scientific discipline (academia), discipline, overlapping with biology, chemistry, pharmacology, and medicine, that involves the study of the adverse effects of chemical substances on living organisms and the practice of diagnos ...
issues prevented long term use of clomifene and further drug development for other potential applications such as breast cancer
Breast cancer is a cancer that develops from breast tissue. Signs of breast cancer may include a Breast lump, lump in the breast, a change in breast shape, dimpling of the skin, Milk-rejection sign, milk rejection, fluid coming from the nipp ...
treatment and prevention.
It was another ten years before tamoxifen was approved in December 1977, not as a contraceptive but as a hormonal treatment to treat and prevent breast cancer. The discovery in 1987 that the SERMs tamoxifen and raloxifene, then thought to be antiestrogen
Antiestrogens, also known as estrogen antagonists or estrogen blockers, are a class of drugs which prevent estrogens like estradiol from mediating their biological effects in the body. They act by blocking the estrogen receptor (ER) and/or inh ...
s because of antagonist effects in breast tissue, showed estrogenic effects in preventing bone loss in ovariectomized rats had a great effect on our understanding of the function of estrogen receptors and nuclear receptor
In the field of molecular biology, nuclear receptors are a class of proteins responsible for sensing steroids, thyroid hormones, vitamins, and certain other molecules. These intracellular receptors work with other proteins to regulate the ex ...
s in general. The term SERM was introduced to describe these compounds that have a combination of estrogen agonist
An agonist is a chemical that activates a Receptor (biochemistry), receptor to produce a biological response. Receptors are Cell (biology), cellular proteins whose activation causes the cell to modify what it is currently doing. In contrast, an R ...
, partial agonist, or antagonist activities depending on the tissue. Toremifene has been shown to be compatible with tamoxifen, and in 1996 it was approved for use in the treatment of breast cancer in postmenopausal women.
Raloxifene originally failed as a breast cancer drug due to its poor performance in comparison to tamoxifen in the laboratory but the estrogenic effects of raloxifene on bone led to its rediscovery and approval in 1997. It was approved for prevention and treatment of osteoporosis and was the first clinically available SERM to prevent both osteoporosis and breast cancer. Ospemifene was approved on February 26, 2013, for the treatment of moderate to severe dyspareunia
Dyspareunia ( ) is painful sexual intercourse due to somatic or psychological causes. The term ''dyspareunia'' covers both female dyspareunia and male dyspareunia, but many discussions that use the term without further specification concern the f ...
, which is a symptom, due to menopause
Menopause, also known as the climacteric, is the time when Menstruation, menstrual periods permanently stop, marking the end of the Human reproduction, reproductive stage for the female human. It typically occurs between the ages of 45 and 5 ...
, of vulvar and vaginal atrophy
Atrophy is the partial or complete wasting away of a part of the body. Causes of atrophy include mutations (which can destroy the gene to build up the organ), malnutrition, poor nourishment, poor circulatory system, circulation, loss of hormone, ...
. Combined therapy with conjugated estrogens and the SERM bazedoxifene, was approved on October 3, 2013, for the treatment of vasomotor symptoms linked with menopause. Bazedoxifene is also used in the prevention of postmenopausal osteoporosis. The search for a potent SERM with bone efficacy and better bioavailability than raloxifene led to the discovery of lasofoxifene. Although lasofoxifene was approved in 2009, it was not marketed for three years following the approval, so the marketing authorization for it has expired. In Europe, bazedoxifene is indicated for the treatment of osteoporosis in postmenopausal women at increased risk of fracture. In India, ormeloxifene
Ormeloxifene, also known as centchroman, is one of the selective estrogen receptor modulators, or SERMs, a class of medication which acts on the estrogen receptor. It is best known as a nonsteroidal oral contraceptive which is taken once per w ...
has been used for dysfunctional uterine bleeding
Abnormal uterine bleeding is vaginal bleeding from the uterus that is abnormally frequent, lasts excessively long, is heavier than normal, or is irregular. The term "dysfunctional uterine bleeding" was used when no underlying cause was presen ...
and birth control.
See also
* Estrogen deprivation therapy * List of selective estrogen receptor modulators *Selective androgen receptor modulator
Selective androgen receptor modulators (SARMs) are a class of drugs that tissue selectivity, selectively activate the androgen receptor in specific tissue (biology), tissues, promoting muscle and bone growth while having less effect on male re ...
* Selective estrogen receptor degrader
* Selective receptor modulator
In the field of pharmacology, a selective receptor modulator or SRM is a type of drug that has different effects in different tissues. An SRM may behave as an agonist in some tissues while as an antagonist in others. Hence selective receptor mod ...
* Timeline of cancer treatment development
References
External links
AACR Cancer Concepts Factsheet on SERMs
* ttp://www.femarelle.com Femarelle official site
Raloxifene (Evista) official site
{{Estrogen receptor modulators Hormonal antineoplastic drugs Progonadotropins