Salen Complex on:
[Wikipedia]
[Google]
[Amazon]
A metal salen complex is a  The metal-free salen compound (H2salen or salenH2) has two
The metal-free salen compound (H2salen or salenH2) has two
 Salen complexes with ''d''8 metal ions, such as Ni(salen), typically have a
Salen complexes with ''d''8 metal ions, such as Ni(salen), typically have a

 Unsubstituted salen complexes are poorly soluble in
Unsubstituted salen complexes are poorly soluble in 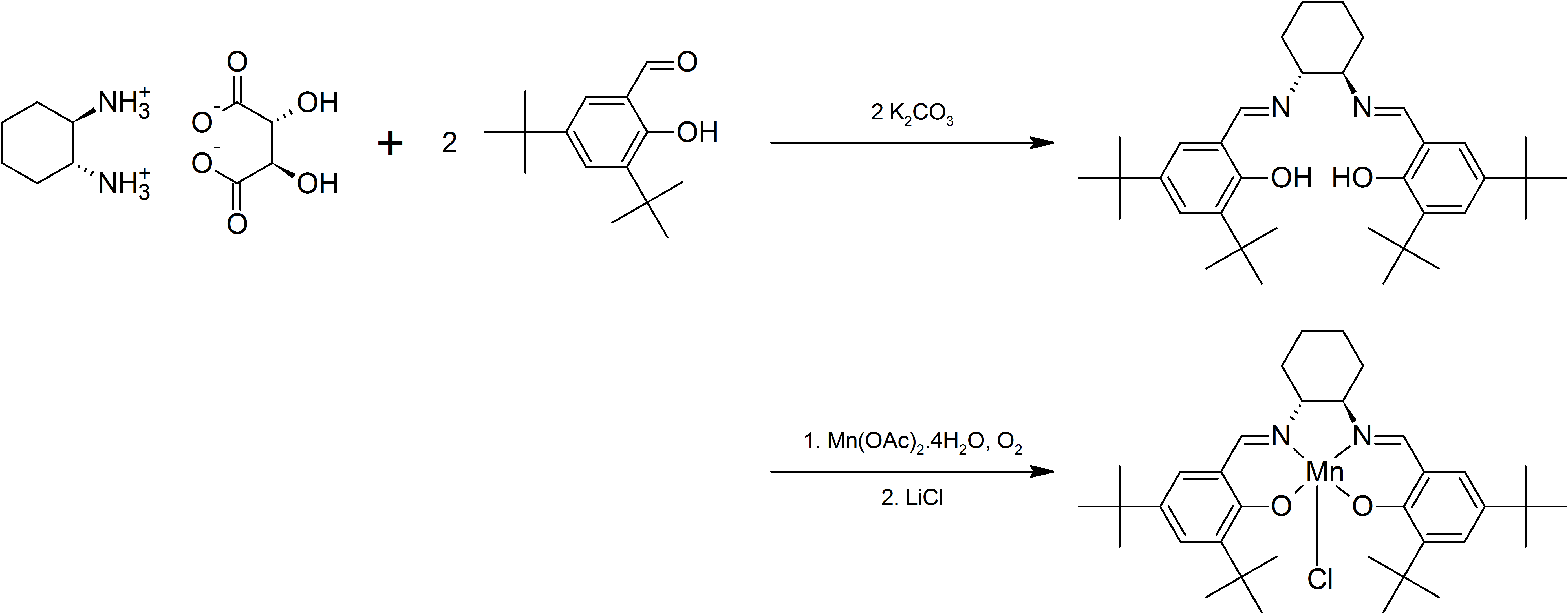
coordination compound
A coordination complex consists of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or complexing agents. Many ...
between a metal cation
An ion () is an atom or molecule with a net electrical charge.
The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by conve ...
and a ligand
In coordination chemistry, a ligand is an ion or molecule ( functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's ele ...
derived from ''N'',''N''′-bis(salicylidene)ethylenediamine, commonly called salen. The classical example is salcomine, the complex with divalent
In chemistry, the valence (US spelling) or valency (British spelling) of an element is the measure of its combining capacity with other atoms when it forms chemical compounds or molecules.
Description
The combining capacity, or affinity of a ...
cobalt
Cobalt is a chemical element with the symbol Co and atomic number 27. As with nickel, cobalt is found in the Earth's crust only in a chemically combined form, save for small deposits found in alloys of natural meteoric iron. The free element, ...
, usually denoted as Co(salen). These complexes are widely investigated as catalysts and enzyme mimics.
phenol
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it ...
ic hydroxyl
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydrox ...
groups. The salen ligand
Salen refers to a tetradentate C2-symmetric ligand synthesized from salicylaldehyde (sal) and ethylenediamine (en). It may also refer to a class of compounds, which are structurally related to the classical salen ligand, primarily bis- Schiff ba ...
is usually its conjugate base (salen2−), resulting from the loss of protons from those hydroxyl groups. The metal atom usually makes four coordination bonds to the oxygen
Oxygen is the chemical element with the symbol O and atomic number 8. It is a member of the chalcogen group in the periodic table, a highly reactive nonmetal, and an oxidizing agent that readily forms oxides with most elements as we ...
and nitrogen
Nitrogen is the chemical element with the symbol N and atomic number 7. Nitrogen is a nonmetal and the lightest member of group 15 of the periodic table, often called the pnictogens. It is a common element in the universe, estimated at seve ...
atoms.
Preparation of complexes
The salen anion forms complexes with mosttransition metal
In chemistry, a transition metal (or transition element) is a chemical element in the d-block of the periodic table (groups 3 to 12), though the elements of group 12 (and less often group 3) are sometimes excluded. They are the elements that c ...
s. These complexes are usually prepared by the reaction of H2salen ("proligand") with metal precursors containing built-in bases, such as alkoxides, metal amides Metal amides (systematic name metal azanides) are a class of coordination compounds composed of a metal center with amide ligands of the form NR2−. Amide ligands have two electron pairs available for bonding. In principle, they can be terminal or ...
, or metal acetate. The proligand may also be treated with a metal halide
Metal halides are compounds between metals and halogens. Some, such as sodium chloride are ionic, while others are covalently bonded. A few metal halides are discrete molecules, such as uranium hexafluoride, but most adopt polymeric structures, s ...
, with or without an added base. Lastly, the proligand may be deprotonated by a nonnucleophilic base, such as sodium hydride
Sodium hydride is the chemical compound with the empirical formula Na H. This alkali metal hydride is primarily used as a strong yet combustible base in organic synthesis. NaH is a saline (salt-like) hydride, composed of Na+ and H− ions, in ...
, before treatment with the metal halide. For example, Jacobsen's catalyst is prepared from the salen ligand precursor with manganese acetate.
Structures
low-spin Spin states when describing transition metal coordination complexes refers to the potential spin configurations of the central metal's d electrons. For several oxidation states, metals can adopt high-spin and low-spin configurations. The ambiguity o ...
square planar molecular geometry
The square planar molecular geometry in chemistry describes the stereochemistry (spatial arrangement of atoms) that is adopted by certain chemical compounds. As the name suggests, molecules of this geometry have their atoms positioned at the corn ...
in the coordination sphere
In coordination chemistry, the first coordination sphere refers to the array of molecules and ions (the ligands) directly attached to the central metal atom. The second coordination sphere consists of molecules and ions that attached in various ...
.
Other metal–salen complexes may have additional ligands above the salen nitrogen–oxygen plane. Complexes with one extra ligand, such as VO(salen), may have a square pyramidal molecular geometry
In molecular geometry, square pyramidal geometry describes the shape of certain compounds with the formula where L is a ligand. If the ligand atoms were connected, the resulting shape would be that of a pyramid with a square base. The point ...
. Complexes with two extra ligands, such as Co(salen)Cl( py), may have octahedral geometry
In chemistry, octahedral molecular geometry, also called square bipyramidal, describes the shape of compounds with six atoms or groups of atoms or ligands symmetrically arranged around a central atom, defining the vertices of an octahedron. The o ...
. Usually the MN2O2 core is relatively planar, even though the ethylene backbone is skewed and the overall salen ligand takes a twisted C2 symmetry. Examples exist where ancillary ligands force the N2O2 donors out of planarity. No evidence indicates that salen is a redox-noninnocent ligand
In chemistry, a (redox) non-innocent ligand is a ligand in a metal complex where the oxidation state is not
clear. Typically, complexes containing non-innocent ligands are redox active at mild potentials. The concept assumes that redox reaction ...
.
Reactions
Enzyme mimics
Tsumaki described the first metal–salen complexes in 1938. He found that the cobalt(II) complex Co(salen) reversibly binds O2, which led to intensive research on cobalt complexes of salen and relatedligand
In coordination chemistry, a ligand is an ion or molecule ( functional group) that binds to a central metal atom to form a coordination complex. The bonding with the metal generally involves formal donation of one or more of the ligand's ele ...
s for their capacity for oxygen storage and transport, looking for potential synthetic oxygen carriers. Cobalt salen complexes also replicate certain aspects of vitamin B12.
Homogeneous catalysis
The manganese-containing salen complex catalyzes the asymmetric epoxidation ofalkene
In organic chemistry, an alkene is a hydrocarbon containing a carbon–carbon double bond.
Alkene is often used as synonym of olefin, that is, any hydrocarbon containing one or more double bonds.H. Stephen Stoker (2015): General, Organic, an ...
s. In the hydrolytic kinetic resolution technique, a racemic
In chemistry, a racemic mixture, or racemate (), is one that has equal amounts of left- and right-handed enantiomers of a chiral molecule or salt. Racemic mixtures are rare in nature, but many compounds are produced industrially as racemates. ...
mixture of epoxides
In organic chemistry, an epoxide is a cyclic ether () with a three-atom ring. This ring approximates an equilateral triangle, which makes it strained, and hence highly reactive, more so than other ethers. They are produced on a large scale f ...
may be separated by selectively hydrolyzing
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution, elimination, and solvation reactions in which water is the nucleophile.
Biological hydrolysis i ...
one enantiomer
In chemistry, an enantiomer ( /ɪˈnænti.əmər, ɛ-, -oʊ-/ ''ih-NAN-tee-ə-mər''; from Ancient Greek ἐνάντιος ''(enántios)'' 'opposite', and μέρος ''(méros)'' 'part') – also called optical isomer, antipode, or optical ant ...
, catalyzed by the analogous cobalt(III) complex. In subsequent work, chromium(III) and cobalt(III) salen complexes catalyze the reaction of carbon dioxide
Carbon dioxide ( chemical formula ) is a chemical compound made up of molecules that each have one carbon atom covalently double bonded to two oxygen atoms. It is found in the gas state at room temperature. In the air, carbon dioxide is t ...
and epoxides to give polycarbonates
Polycarbonates (PC) are a group of thermoplastic polymers containing carbonate groups in their chemical structures. Polycarbonates used in engineering are strong, tough materials, and some grades are optically transparent. They are easily worke ...
.
Related complexes
Substituted salen complexes
organic solvents
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for ...
. Side chains attached to the ethylene bridge or the benzene rings may increase the solubility
In chemistry, solubility is the ability of a substance, the solute, to form a solution with another substance, the solvent. Insolubility is the opposite property, the inability of the solute to form such a solution.
The extent of the solub ...
. An example is the salpn ligand, derived from 1,2-diaminopropane instead of ethylenediamine
Ethylenediamine (abbreviated as en when a ligand) is the organic compound with the formula C2H4(NH2)2. This colorless liquid with an ammonia-like odor is a basic amine. It is a widely used building block in chemical synthesis, with approximately ...
, which is used as a metal deactivating additive in motor oil
Motor oil, engine oil, or engine lubricant is any one of various substances used for the lubrication of internal combustion engines. They typically consist of base oils enhanced with various additives, particularly antiwear additives, deterg ...
s and motor fuel
An engine or motor is a machine designed to convert one or more forms of energy into mechanical energy.
Available energy sources include potential energy (e.g. energy of the Earth's gravitational field as exploited in hydroelectric power ...
.
The presence of bulky groups near the coordination site is generally desirable, as it enhances catalytic activity and prevents dimerization. Salen ligands derived from 3,5-di-''tert''-butylsalicylaldehyde are popular because they fulfill both criteria, and tend to be soluble even in non-polar solvents
In chemistry, polarity is a separation of electric charge leading to a molecule or its chemical groups having an electric dipole moment, with a negatively charged end and a positively charged end.
Polar molecules must contain one or more pola ...
like pentane
Pentane is an organic compound with the formula C5H12—that is, an alkane with five carbon atoms. The term may refer to any of three structural isomers, or to a mixture of them: in the IUPAC nomenclature, however, pentane means exclusively the ' ...
.
Chirality
Chirality is a property of asymmetry important in several branches of science. The word ''chirality'' is derived from the Greek (''kheir''), "hand", a familiar chiral object.
An object or a system is ''chiral'' if it is distinguishable from i ...
may be introduced into the ligand either via the diamine backbone, via the phenyl ring
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula C6 H5, and is often represented by the symbol Ph. Phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydrogen ...
, or both. For example, condensation
Condensation is the change of the state of matter from the gas phase into the liquid phase, and is the reverse of vaporization. The word most often refers to the water cycle. It can also be defined as the change in the state of water vapor ...
of the C2-symmetric ''trans''-1,2-diaminocyclohexane
''trans''-1,2-Diaminocyclohexane is an organic compound with the formula C6H10(NH2)2. This diamine is a building block for ''C''2-symmetric ligands that are useful in asymmetric catalysis.
A mixture of all three stereoisomers of 1,2-diamino ...
with 3,5-di-''tert''-butylsalicylaldehdye gives a ligand that forms complexes with Cr, Mn, Co, Al, which have proven useful for asymmetric transformations. For an example, see the Jacobsen epoxidation
The Jacobsen epoxidation, sometimes also referred to as Jacobsen-Katsuki epoxidation is a chemical reaction which allows enantioselective epoxidation of unfunctionalized alkyl- and aryl- substituted alkenes. It is complementary to the Sharpless ...
, which is catalyzed by a chiral manganese
Manganese is a chemical element with the symbol Mn and atomic number 25. It is a hard, brittle, silvery metal, often found in minerals in combination with iron. Manganese is a transition metal with a multifaceted array of industrial alloy u ...
-salen complex:
:
Complexes with salen-type ligands
"Salen-type" metal complexes are formed with ligands with similar chelating groups, such as acacen, salph, and salqu. Salqu copper complexes have been investigated as oxidation catalysts.Complexes with salan ligands
Complexes with the similarsalan
]
Salan, Salanus or Zalan ( Bulgarian language, Bulgarian and Serbian Cyrillic: Салан or Залан; hu, Zalán; ro, Salanus) was, according to the Gesta Hungarorum, a local Bulgarianhttp://keptar.niif.hu/000500/000586/magyaro-honf-terke ...
or salalen ligand, salalen ligands, with one or two saturated nitrogen–aryl bonds (amine
In chemistry, amines (, ) are compounds and functional groups that contain a basic nitrogen atom with a lone pair. Amines are formally derivatives of ammonia (), wherein one or more hydrogen atoms have been replaced by a substituent su ...
s rather than imine
In organic chemistry, an imine ( or ) is a functional group or organic compound containing a carbon–nitrogen double bond (). The nitrogen atom can be attached to a hydrogen or an organic group (R). The carbon atom has two additional single bo ...
s) tend to be less rigid and more electron-rich at the metal center than the corresponding salen complexes.
Further reading
* *References
{{Reflist Coordination chemistry Organometallic chemistry Ligands