SN1 reaction on:
[Wikipedia]
[Google]
[Amazon]
 The unimolecular nucleophilic substitution (SN1) reaction is a
The unimolecular nucleophilic substitution (SN1) reaction is a
 This SN1 reaction takes place in three steps:
* Formation of a ''tert''-butyl carbocation by separation of a
This SN1 reaction takes place in three steps:
* Formation of a ''tert''-butyl carbocation by separation of a  :
: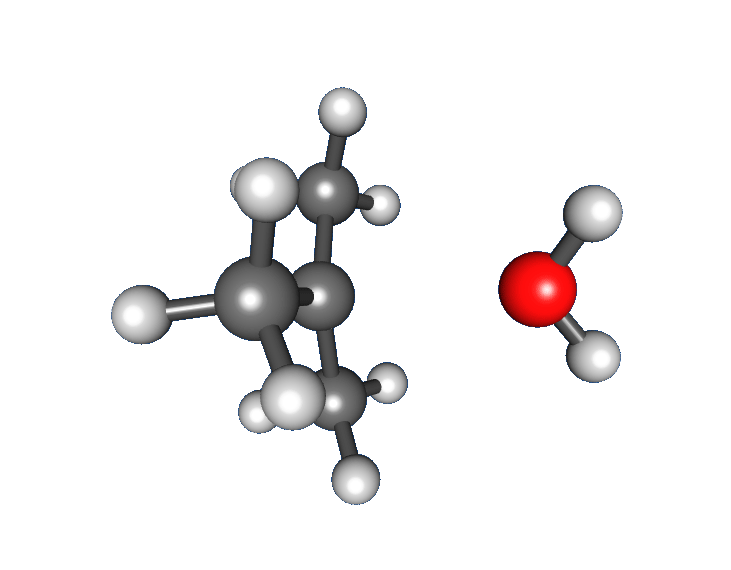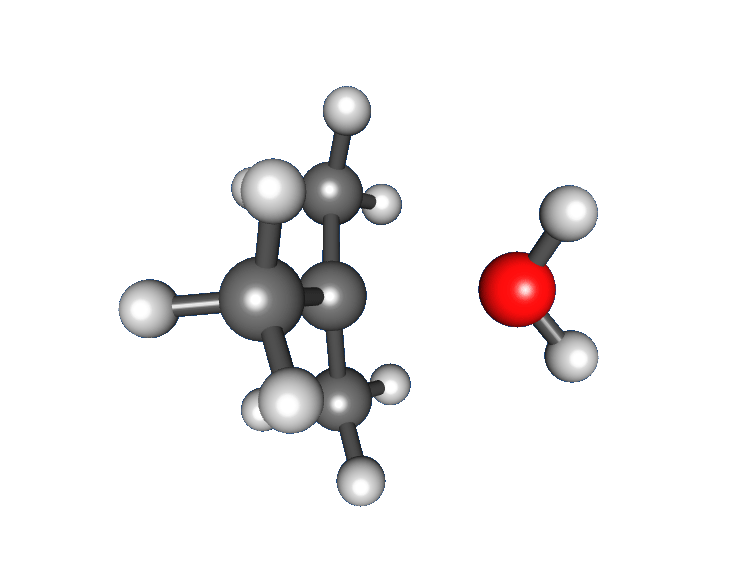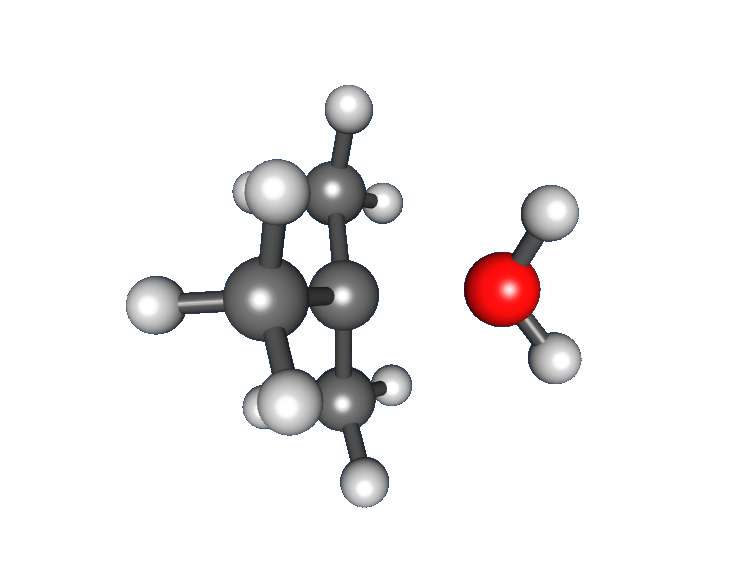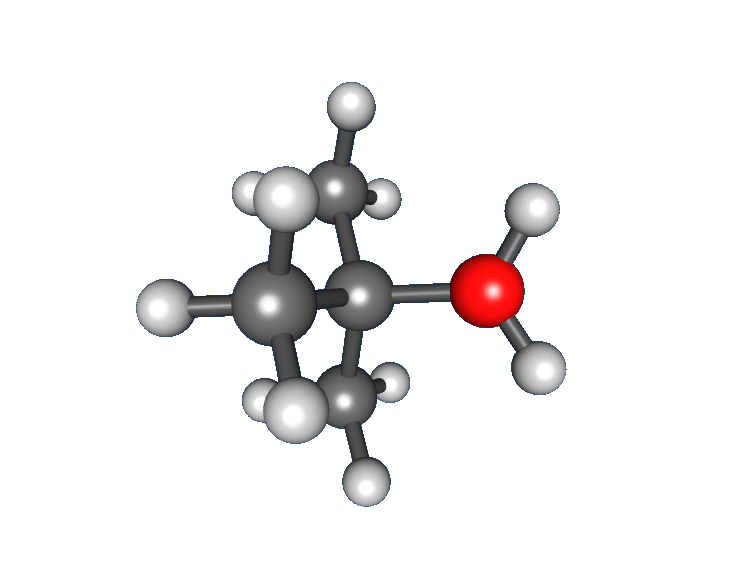 * Nucleophilic attack: the carbocation reacts with the nucleophile. If the
* Nucleophilic attack: the carbocation reacts with the nucleophile. If the  *
* 
 Though a relatively stable tertiary
Though a relatively stable tertiary
substitution reaction
A substitution reaction (also known as single displacement reaction or single substitution reaction) is a chemical reaction during which one functional group in a chemical compound is replaced by another functional group. Substitution reactions ar ...
in organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
. The Hughes-Ingold symbol of the mechanism expresses two properties—"SN" stands for "nucleophilic substitution
In chemistry, a nucleophilic substitution (SN) is a class of chemical reactions in which an electron-rich chemical species (known as a nucleophile) replaces a functional group within another electron-deficient molecule (known as the electrophile) ...
", and the "1" says that the rate-determining step is unimolecular. Thus, the rate equation
In chemistry, the rate equation (also known as the rate law or empirical differential rate equation) is an Empirical relationship, empirical Differential equation, differential Expression (mathematics), mathematical expression for the reaction rat ...
is often shown as having first-order dependence on the substrate and zero-order dependence on the nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
. This relationship holds for situations where the amount of nucleophile is much greater than that of the intermediate. Instead, the rate equation may be more accurately described using steady-state kinetics. The reaction involves a carbocation
Carbocation is a general term for ions with a positively charged carbon atom. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further ...
intermediate and is commonly seen in reactions of secondary or tertiary alkyl halides under strongly basic conditions or, under strongly acidic conditions, with secondary or tertiary alcohols. With primary and secondary alkyl halides, the alternative SN2 reaction occurs. In inorganic chemistry
Inorganic chemistry deals with chemical synthesis, synthesis and behavior of inorganic compound, inorganic and organometallic chemistry, organometallic compounds. This field covers chemical compounds that are not carbon-based, which are the subj ...
, the SN1 reaction is often known as the ''dissociative substitution
In chemistry, dissociative substitution describes a reaction pathway by which compounds interchange ligands. The term is typically applied to coordination and organometallic complexes, but resembles the SN1 mechanism in organic chemistry. Thi ...
''. This dissociation pathway is well-described by the cis effect
In inorganic chemistry, the cis effect is defined as the labilization (or destabilization) of CO ligands that are ''cis'' to other ligands. CO is a well-known strong pi-accepting ligand in organometallic chemistry that will labilize in the ''cis ...
. A reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical reaction occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage ...
was first introduced by Christopher Ingold et al. in 1940. This reaction does not depend much on the strength of the nucleophile, unlike the SN2 mechanism. This type of mechanism involves two steps. The first step is the ionization of alkyl halide in the presence of aqueous acetone or ethyl alcohol. This step provides a carbocation as an intermediate.
In the first step of SN1 mechanism, a carbocation is formed which is planar and hence attack of nucleophile (second step) may occur from either side to give a racemic product, but actually complete racemization does not take place. This is because the nucleophilic species attacks the carbocation even before the departing halides ion has moved sufficiently away from the carbocation. The negatively charged halide ion shields the carbocation from being attacked on the front side, and backside attack, which leads to inversion of configuration, is preferred. Thus the actual product no doubt consists of a mixture of enantiomers but the enantiomers with inverted configuration would predominate and complete racemization does not occur.
Mechanism
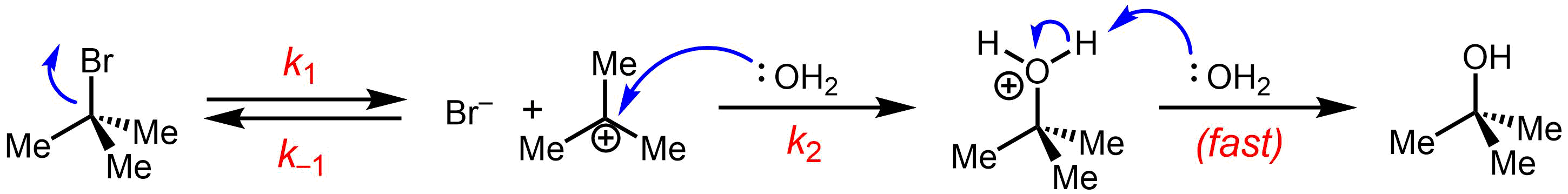
An example of a reaction taking place with an SN1reaction mechanism
In chemistry, a reaction mechanism is the step by step sequence of elementary reactions by which overall chemical reaction occurs.
A chemical mechanism is a theoretical conjecture that tries to describe in detail what takes place at each stage ...
is the hydrolysis
Hydrolysis (; ) is any chemical reaction in which a molecule of water breaks one or more chemical bonds. The term is used broadly for substitution reaction, substitution, elimination reaction, elimination, and solvation reactions in which water ...
of tert-butyl bromide forming ''tert''-butanol:
:leaving group
In organic chemistry, a leaving group typically means a Chemical species, molecular fragment that departs with an electron, electron pair during a reaction step with heterolysis (chemistry), heterolytic bond cleavage. In this usage, a ''leaving gr ...
(a bromide
A bromide ion is the negatively charged form (Br−) of the element bromine, a member of the halogens group on the periodic table. Most bromides are colorless. Bromides have many practical roles, being found in anticonvulsants, flame-retard ...
anion) from the carbon atom: this step is slow.
: * Nucleophilic attack: the carbocation reacts with the nucleophile. If the
* Nucleophilic attack: the carbocation reacts with the nucleophile. If the nucleophile
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they are ...
is a neutral molecule (i.e. a solvent
A solvent (from the Latin language, Latin ''wikt:solvo#Latin, solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a Solution (chemistry), solution. A solvent is usually a liquid but can also be a solid, a gas ...
) a third step is required to complete the reaction. When the solvent is water, the intermediate is an oxonium ion. This reaction step is fast.
:Deprotonation
Deprotonation (or dehydronation) is the removal (transfer) of a proton (or hydron, or hydrogen cation), (H+) from a Brønsted–Lowry acid in an acid–base reaction.Henry Jakubowski, Biochemistry Online Chapter 2A3, https://employees.csbsju.ed ...
: Removal of a proton on the protonated nucleophile by water acting as a base forming the alcohol
Alcohol may refer to:
Common uses
* Alcohol (chemistry), a class of compounds
* Ethanol, one of several alcohols, commonly known as alcohol in everyday life
** Alcohol (drug), intoxicant found in alcoholic beverages
** Alcoholic beverage, an alco ...
and a hydronium ion
In chemistry, hydronium (hydroxonium in traditional British English) is the cation , also written as , the type of oxonium ion produced by protonation of water. It is often viewed as the positive ion present when an Arrhenius acid is dissolved in ...
. This reaction step is fast.
Rate law
Although the rate law of the SN1 reaction is often regarded as being first order in alkyl halide and zero order in nucleophile, this is a simplification that holds true only under certain conditions. While it, too, is an approximation, the rate law derived from the steady state approximation (SSA) provides more insight into the kinetic behavior of the SN1 reaction. Consider the following reaction scheme for the mechanism shown above: Though a relatively stable tertiary
Though a relatively stable tertiary carbocation
Carbocation is a general term for ions with a positively charged carbon atom. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further ...
, ''tert''-butyl cation is a high-energy species that is present only at very low concentration and cannot be directly observed under normal conditions. Thus, the SSA can be applied to this species:
(1) Steady state assumption:
(2) Concentration of t-butyl cation, based on steady state assumption:
(3) Overall reaction rate, assuming rapid final step:
(4) Steady state rate law, by plugging (2) into (3):
Under normal synthetic conditions, the entering nucleophile is more nucleophilic than the leaving group and is present in excess. Moreover, kinetic experiments are often conducted under initial rate conditions (5 to 10% conversion) and without the addition of bromide, so