Ronald Breslow on:
[Wikipedia]
[Google]
[Amazon]
Ronald Charles David Breslow (March 14, 1931 – October 25, 2017) was an American
chemist
A chemist (from Greek ''chēm(ía)'' alchemy; replacing ''chymist'' from Medieval Latin ''alchemist'') is a graduated scientist trained in the study of chemistry, or an officially enrolled student in the field. Chemists study the composition of ...
from Rahway, New Jersey
Rahway () is a city (New Jersey), city in southern Union County, New Jersey, Union County, in the U.S. state of New Jersey. A bedroom community of New York City, it is centrally located in the Rahway River, Rahway Valley region, in the New ...
. He was University Professor
Professor (commonly abbreviated as Prof.) is an academic rank at universities and other post-secondary education and research institutions in most countries. Literally, ''professor'' derives from Latin as a 'person who professes'. Professors ...
at Columbia University
Columbia University in the City of New York, commonly referred to as Columbia University, is a Private university, private Ivy League research university in New York City. Established in 1754 as King's College on the grounds of Trinity Churc ...
, where he was based in the Department of Chemistry and affiliated with the Departments of Biological Sciences and Pharmacology; he had also been on the faculty of its Department of Chemical Engineering. He had taught at Columbia since 1956 and was a former chair of the university's chemistry department.
Life and career
Breslow was born in Rahway, New Jersey, the son of Gladys (Fellows) and Alexander E. Breslow. He was interested in the design and synthesis of new molecules with interesting properties, and the study of these properties. Examples include the cyclopropenylcation
An ion () is an atom or molecule with a net electrical charge. The charge of an electron is considered to be negative by convention and this charge is equal and opposite to the charge of a proton, which is considered to be positive by convent ...
, the simplest aromatic
In organic chemistry, aromaticity is a chemical property describing the way in which a conjugated system, conjugated ring of unsaturated bonds, lone pairs, or empty orbitals exhibits a stabilization stronger than would be expected from conjugati ...
system and the first aromatic compound prepared with other than six electrons
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
in a ring. His seminal contributions include the correct site of reactivity of thiamin diphosphate in enzymes that promote the decarboxylation of pyruvate – based on his pioneering use of proton NMR with small molecule analogues – and the rate enhancement provided by binding to cyclodextrins produced major themes for study in modern organic and biological chemistry. He also co-discovered the histone deacetylase inhibitor SAHA (Vorinostat
Vorinostat (International Nonproprietary Name, rINN), also known as suberoylanilide hydroxamic acid (suberic acid, suberoyl+aniline, anilide+hydroxamic acid abbreviated as SAHA), is a member of a larger class of compounds that inhibit histone de ...
) which is FDA-approved for the treatment of cutaneous T-cell lymphoma.
Breslow earned his B.A., M.A. and Ph.D. from Harvard University
Harvard University is a Private university, private Ivy League research university in Cambridge, Massachusetts, United States. Founded in 1636 and named for its first benefactor, the History of the Puritans in North America, Puritan clergyma ...
, where his doctoral advisor was R. B. Woodward. Among Breslow's former Ph.D. students is Robert Grubbs, who won the Nobel Prize in Chemistry in 2005, and Doug La Follette
Douglas J. La Follette (born June 6, 1940) is a retired American academic, environmental scientist, and Democratic politician from Wisconsin. He was the 28th and 30th secretary of state of Wisconsin, serving from 1975 to 1979, and from 1983 to ...
, Secretary of State of Wisconsin. Robert Lefkowitz, who won the Nobel Prize in Chemistry in 2012, studied under Breslow as an undergraduate.
Breslow received many honors and awards, including the National Medal of Science
The National Medal of Science is an honor bestowed by the President of the United States to individuals in science and engineering who have made important contributions to the advancement of knowledge in the fields of behavioral science, behavior ...
in 1991, the Welch Award, the Arthur C. Cope Award (1987), the NAS Award in Chemical Sciences
The National Academy of Sciences Award in Chemical Sciences is awarded for innovative research in the chemical sciences that in the broadest sense contributes to a better understanding of the natural sciences and to the benefit of humanity.
Recip ...
, the American Chemical Society's ACS Award in Pure Chemistry (1966), the Othmer Gold Medal
The Othmer Gold Medal recognizes outstanding individuals who contributed to progress in chemistry and science through their activities in areas including innovation, entrepreneurship, research, education, public understanding, legislation, and ph ...
(2006), the Priestley Medal (1999), and the 2014 American Institute of Chemists (AIC) Gold Medal. In recognition of his classroom skills, Columbia awarded him both its Mark Van Doren Award and its Great Teacher Award. He served as president of the ACS in 1996 and chaired the chemistry division of the National Academy of Sciences
The National Academy of Sciences (NAS) is a United States nonprofit, NGO, non-governmental organization. NAS is part of the National Academies of Sciences, Engineering, and Medicine, along with the National Academy of Engineering (NAE) and the ...
from 1974 to 1977. In 1997 he was named one of the top 75 contributors to the chemical enterprise of the past 75 years by Chemical & Engineering News
''Chemical & Engineering News'' (''C&EN'') is a weekly news magazine published by the American Chemical Society (ACS), providing professional and technical news and analysis in the fields of chemistry and chemical engineering.Myron L. Bender distinguished lecturer at
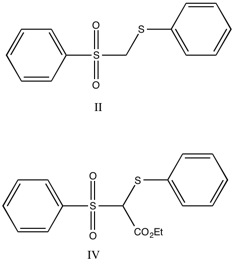 Compound II was treated with
Compound II was treated with 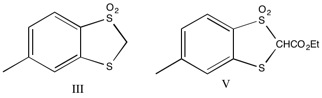 The ester analogs were prepared (IV and V) and were found to be acidic enough for titration. The compounds were titrated under nitrogen with 0.2N NaOH with a Beckman Model GS meter with an E-2 electrode. Compound IV was found to have a pKa of 8.9 +/- 0.1, while compound V had a pKa of 11.1 +/- 0.2.
The ester analogs were prepared (IV and V) and were found to be acidic enough for titration. The compounds were titrated under nitrogen with 0.2N NaOH with a Beckman Model GS meter with an E-2 electrode. Compound IV was found to have a pKa of 8.9 +/- 0.1, while compound V had a pKa of 11.1 +/- 0.2.
 However, there is considerable debate as to how the L conformation of α-methyl amino acids was selected for. The most widely accepted theory is that right circularly polarized light in outer space (somewhat) selectively destroyed the D conformation. In theory,
However, there is considerable debate as to how the L conformation of α-methyl amino acids was selected for. The most widely accepted theory is that right circularly polarized light in outer space (somewhat) selectively destroyed the D conformation. In theory,  Note that the above product can be protonated from either face with equal probability. The final acid is generated by hydrolysis of the imine.
It's worth noting that the alpha keto acid is believed to be formed from a Strecker-like reaction, shown below.
Note that the above product can be protonated from either face with equal probability. The final acid is generated by hydrolysis of the imine.
It's worth noting that the alpha keto acid is believed to be formed from a Strecker-like reaction, shown below.
 From figure 5, we see that the L alpha-methyl amino acids do not directly act as a chiral directing group to generate the normal L amino acid. Researchers hoped that a second molecule of the alpha-methy amino acid could act as a directing group, however they found that the D enantiomer was slightly favored when only L alpha-methyl amino acids were present. The figure below shows how the D enantiomer is favored.
From figure 5, we see that the L alpha-methyl amino acids do not directly act as a chiral directing group to generate the normal L amino acid. Researchers hoped that a second molecule of the alpha-methy amino acid could act as a directing group, however they found that the D enantiomer was slightly favored when only L alpha-methyl amino acids were present. The figure below shows how the D enantiomer is favored.
 When researchers added copper to the reaction, the resulting product was the L enantiomer. Meteors have been found to contain both copper and zinc-justifying the researchers' use of the metal. However, when zinc was used in the same reaction, the L enantiomer was not preferentially formed. Based on computational calculations, the copper forms a square planar complex (shown below) and sterics facilitate protonation to generate the L amino acid.
When researchers added copper to the reaction, the resulting product was the L enantiomer. Meteors have been found to contain both copper and zinc-justifying the researchers' use of the metal. However, when zinc was used in the same reaction, the L enantiomer was not preferentially formed. Based on computational calculations, the copper forms a square planar complex (shown below) and sterics facilitate protonation to generate the L amino acid.
 When a slight enantiomeric excess is present, the solubility's of the pure and racemic crystal can be manipulated to generate large ee's of the pure enantiomer. If we define certain solubility's as such:
KL= represents the solubility of the pure enantiomer
KDL= represents the solubility product of the racemic mixture such that
KDL/ We can then define the ratio of = /KDL
When both enantiomers are present, a racemic crystal structure is formed-however it is lower in energy, has a higher melting point, and is less soluble than the enantiomerically pure crystal structure. As a result, when a slight excess of one enantiomer is present, the ee can be amplified by evaporating solvent-causing the racemate to precipitate. Researchers have been able to start with an ee of 1% L, and ultimately end up with 95:5 solution of L: D. The results discussed above (particularly the synchrotron argument) led Breslow to propose that the D amino acids and L sugars could generate life in other parts of the universe.
When a slight enantiomeric excess is present, the solubility's of the pure and racemic crystal can be manipulated to generate large ee's of the pure enantiomer. If we define certain solubility's as such:
KL= represents the solubility of the pure enantiomer
KDL= represents the solubility product of the racemic mixture such that
KDL/ We can then define the ratio of = /KDL
When both enantiomers are present, a racemic crystal structure is formed-however it is lower in energy, has a higher melting point, and is less soluble than the enantiomerically pure crystal structure. As a result, when a slight excess of one enantiomer is present, the ee can be amplified by evaporating solvent-causing the racemate to precipitate. Researchers have been able to start with an ee of 1% L, and ultimately end up with 95:5 solution of L: D. The results discussed above (particularly the synchrotron argument) led Breslow to propose that the D amino acids and L sugars could generate life in other parts of the universe.
Official biographyA Video interview of Professor Breslow
{{DEFAULTSORT:Breslow, Ronald 1931 births 2017 deaths People from Rahway, New Jersey Columbia University faculty National Medal of Science laureates Foreign members of the Royal Society Members of the United States National Academy of Sciences Foreign fellows of the Indian National Science Academy Harvard University alumni Members of the American Philosophical Society
Northwestern University
Northwestern University (NU) is a Private university, private research university in Evanston, Illinois, United States. Established in 1851 to serve the historic Northwest Territory, it is the oldest University charter, chartered university in ...
in 1999. The Ronald Breslow Award for Achievement in Biomimetic Chemistry, awarded annually by the ACS, is named in his honor.
He was a member of the National Academy of Sciences
The National Academy of Sciences (NAS) is a United States nonprofit, NGO, non-governmental organization. NAS is part of the National Academies of Sciences, Engineering, and Medicine, along with the National Academy of Engineering (NAE) and the ...
, the American Academy of Arts and Sciences
The American Academy of Arts and Sciences (The Academy) is one of the oldest learned societies in the United States. It was founded in 1780 during the American Revolution by John Adams, John Hancock, James Bowdoin, Andrew Oliver, and other ...
, the European Academy of Sciences, and the American Philosophical Society
The American Philosophical Society (APS) is an American scholarly organization and learned society founded in 1743 in Philadelphia that promotes knowledge in the humanities and natural sciences through research, professional meetings, publicat ...
. He is also a foreign member of the Royal Society
The Royal Society, formally The Royal Society of London for Improving Natural Knowledge, is a learned society and the United Kingdom's national academy of sciences. The society fulfils a number of roles: promoting science and its benefits, re ...
and an honorary member of many other scientific bodies around the world.
In 2012, his paper "Evidence for the Likely Origin of Homochirality in Amino Acids, Sugars, and Nucleosides on Prebiotic Earth" was retracted from the ''Journal of the American Chemical Society
The ''Journal of the American Chemical Society'' (also known as JACS) is a weekly peer-reviewed scientific journal that was established in 1879 by the American Chemical Society. The journal has absorbed two other publications in its history, the ...
'' due to copyright concerns, leading to a debate on self-plagiarism and the distinction between a personal review and a paper.
Synthesis of cyclopropenyl cation
This cation was first prepared by mixing 3-chlorocyclopropene withantimony pentachloride
Antimony pentachloride is a chemical compound with the formula SbCl5. It is a colourless oil, but typical samples are yellowish due to dissolved chlorine. Owing to its tendency to hydrolyse to hydrochloric acid, SbCl5 is a highly corrosive subs ...
, aluminum trichloride, or silver tetrafluoroborate
Silver tetrafluoroborate is an inorganic compound with the molecular formula AgBF4. It is a white solid, although commercial samples often are gray, that dissolves in polar organic solvents as well as water.
Preparation
Silver tetrafluoroborate ca ...
. Carbon-13 NMR
Carbon-13 (C13) nuclear magnetic resonance (most commonly known as carbon-13 NMR spectroscopy or 13C NMR spectroscopy or sometimes simply referred to as carbon NMR) is the application of nuclear magnetic resonance (NMR) spectroscopy to carbon. It ...
shows singlets with a coupling constant of 265 ± 1 Hz. The authors suggest that this coupling constant is suggestive that the C–H bond is 53% s character. The overall bonding framework then consists of sp orbitals to all hydrogens, two sp3 orbitals for each sigma bond
In chemistry, sigma bonds (σ bonds) or sigma overlap are the strongest type of covalent chemical bond. They are formed by head-on overlapping between atomic orbitals along the internuclear axis. Sigma bonding is most simply defined for diat ...
, and one p orbital for the π framework.
D-Orbital Conjugation
It had been suggested that carbanion-sulfone double bonds will not show aromatic character-primarily as a result of the nodes present in d-orbitals. Straight chain analogs were chosen based on the comparable acidity, combined with previous studies indicating that steric effects are largely negligible. The straight chain analogs are shown below. Compound II was treated with
Compound II was treated with dimethoxyethane
Dimethoxyethane, also known as glyme, monoglyme, dimethyl glycol, ethylene glycol dimethyl ether, dimethyl cellosolve, and DME, is a colorless, aprotic, and liquid ether that is used as a solvent, especially in batteries. Dimethoxyethane is mi ...
/ D2O / triethylamine
Triethylamine is the chemical compound with the formula N(CH2CH3)3, commonly abbreviated Et3N. Like triethanolamine and the tetraethylammonium ion, it is often abbreviated TEA. It is a colourless volatile liquid with a strong fishy odor remini ...
and was found to be completely deuterated upon recovery. The deuterated compound was then treated with butyllithium Butyllithium may refer to one of 5 isomeric organolithium reagents of which 3 are commonly used in chemical synthesis:
* ''n''-Butyllithium, abbreviated BuLi or nBuLi
* ''sec''-Butyllithium, abbreviated ''sec''-BuLi or sBuLi, has 2 stereoisomers, ...
in ether, with dimethoxyethane and 2N HCl regenerating the starting material. The cyclic analog (III, shown below) was deuterated in the same manner, and addition of deuterated I, followed by quenching to regenerate the protonated form, was analyzed by NMR. There was one proton peak, and it was shown to equilibrate equally between compounds II and III, indicating that compounds had similar acidity. From this result, the researchers concluded that compound III was not aromatic, because stabilizing effect of aromaticity on the anion should increase the acidity of the parent compound.
 The ester analogs were prepared (IV and V) and were found to be acidic enough for titration. The compounds were titrated under nitrogen with 0.2N NaOH with a Beckman Model GS meter with an E-2 electrode. Compound IV was found to have a pKa of 8.9 +/- 0.1, while compound V had a pKa of 11.1 +/- 0.2.
The ester analogs were prepared (IV and V) and were found to be acidic enough for titration. The compounds were titrated under nitrogen with 0.2N NaOH with a Beckman Model GS meter with an E-2 electrode. Compound IV was found to have a pKa of 8.9 +/- 0.1, while compound V had a pKa of 11.1 +/- 0.2.
Origins of Homochirality on Prebiotic Earth
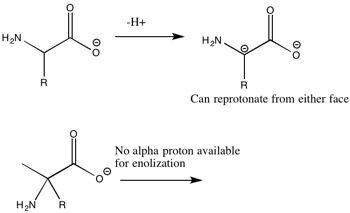
The essential building blocks of life (amino acids and key sugars-ribose and deoxyribose) can exist in one of two forms-L or D. However life has evolved such that the vast majority of amino acids are L and the sugars are D. If the amino acids and sugars present in life were racemic (consisting of both L and D), then proteins, DNA, and RNA would not adopt a well defined conformation, resulting in a loss of function. The question of how this preference first occurred has puzzled scientists for years while several theories have been proposed; there is still no clear answer. There is growing evidence that the chiral preference came from outer space as scientists discovered α-methyl amino acids inside the Murchison meteorite that have a slight enantiomeric excess (ee) for the L conformation. These α-methyl amino acids are believed to be from outer space as a result of their high abundance of 13C and deuterium. Furthermore, α-methyl amino acids are generally not present in terrestrial chemistry. A common critique is that these amino acids would not be able to tolerate the high temperatures upon entering Earth's atmosphere as the meteorite crashed into the planet. However, the amino acids have been found inside the meteorite, with the meteorite acting as an insulator. Unlike regular amino acids, α-methyl amino acids are not capable of racemizing by enolization on an evolutionary time scale, shown below. However, there is considerable debate as to how the L conformation of α-methyl amino acids was selected for. The most widely accepted theory is that right circularly polarized light in outer space (somewhat) selectively destroyed the D conformation. In theory,
However, there is considerable debate as to how the L conformation of α-methyl amino acids was selected for. The most widely accepted theory is that right circularly polarized light in outer space (somewhat) selectively destroyed the D conformation. In theory, synchrotron
A synchrotron is a particular type of cyclic particle accelerator, descended from the cyclotron, in which the accelerating particle beam travels around a fixed closed-loop path. The strength of the magnetic field which bends the particle beam i ...
s produce light of opposite handedness (right and left) above and below the circulation plane. This has been demonstrated in experiments on earth. The theory continues then that neutron stars could act as synchrotrons-with right polarized light pointing in our direction of the universe, and left polarized light pointing in the opposite direction. However, other astronomers claim that the polarization only occurs in the infrared region, which only has sufficient energy to cause molecular vibrations and stretches-far from being capable of destroying molecules.
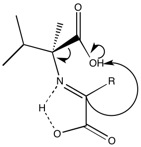
A second problem encountered with the L α-methyl amino acids is how to generate enantiomerically pure regular amino acids from the slight excess. The issue is illustrated in the below figure.
 Note that the above product can be protonated from either face with equal probability. The final acid is generated by hydrolysis of the imine.
It's worth noting that the alpha keto acid is believed to be formed from a Strecker-like reaction, shown below.
Note that the above product can be protonated from either face with equal probability. The final acid is generated by hydrolysis of the imine.
It's worth noting that the alpha keto acid is believed to be formed from a Strecker-like reaction, shown below.
 From figure 5, we see that the L alpha-methyl amino acids do not directly act as a chiral directing group to generate the normal L amino acid. Researchers hoped that a second molecule of the alpha-methy amino acid could act as a directing group, however they found that the D enantiomer was slightly favored when only L alpha-methyl amino acids were present. The figure below shows how the D enantiomer is favored.
From figure 5, we see that the L alpha-methyl amino acids do not directly act as a chiral directing group to generate the normal L amino acid. Researchers hoped that a second molecule of the alpha-methy amino acid could act as a directing group, however they found that the D enantiomer was slightly favored when only L alpha-methyl amino acids were present. The figure below shows how the D enantiomer is favored.
 When researchers added copper to the reaction, the resulting product was the L enantiomer. Meteors have been found to contain both copper and zinc-justifying the researchers' use of the metal. However, when zinc was used in the same reaction, the L enantiomer was not preferentially formed. Based on computational calculations, the copper forms a square planar complex (shown below) and sterics facilitate protonation to generate the L amino acid.
When researchers added copper to the reaction, the resulting product was the L enantiomer. Meteors have been found to contain both copper and zinc-justifying the researchers' use of the metal. However, when zinc was used in the same reaction, the L enantiomer was not preferentially formed. Based on computational calculations, the copper forms a square planar complex (shown below) and sterics facilitate protonation to generate the L amino acid.
 When a slight enantiomeric excess is present, the solubility's of the pure and racemic crystal can be manipulated to generate large ee's of the pure enantiomer. If we define certain solubility's as such:
KL= represents the solubility of the pure enantiomer
KDL= represents the solubility product of the racemic mixture such that
KDL/ We can then define the ratio of = /KDL
When both enantiomers are present, a racemic crystal structure is formed-however it is lower in energy, has a higher melting point, and is less soluble than the enantiomerically pure crystal structure. As a result, when a slight excess of one enantiomer is present, the ee can be amplified by evaporating solvent-causing the racemate to precipitate. Researchers have been able to start with an ee of 1% L, and ultimately end up with 95:5 solution of L: D. The results discussed above (particularly the synchrotron argument) led Breslow to propose that the D amino acids and L sugars could generate life in other parts of the universe.
When a slight enantiomeric excess is present, the solubility's of the pure and racemic crystal can be manipulated to generate large ee's of the pure enantiomer. If we define certain solubility's as such:
KL= represents the solubility of the pure enantiomer
KDL= represents the solubility product of the racemic mixture such that
KDL/ We can then define the ratio of = /KDL
When both enantiomers are present, a racemic crystal structure is formed-however it is lower in energy, has a higher melting point, and is less soluble than the enantiomerically pure crystal structure. As a result, when a slight excess of one enantiomer is present, the ee can be amplified by evaporating solvent-causing the racemate to precipitate. Researchers have been able to start with an ee of 1% L, and ultimately end up with 95:5 solution of L: D. The results discussed above (particularly the synchrotron argument) led Breslow to propose that the D amino acids and L sugars could generate life in other parts of the universe.
References
External links
Official biography
{{DEFAULTSORT:Breslow, Ronald 1931 births 2017 deaths People from Rahway, New Jersey Columbia University faculty National Medal of Science laureates Foreign members of the Royal Society Members of the United States National Academy of Sciences Foreign fellows of the Indian National Science Academy Harvard University alumni Members of the American Philosophical Society