Ring Expansion on:
[Wikipedia]
[Google]
[Amazon]
Ring expansion and ring contraction reactions expand or contract 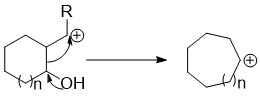
 Rings can be expanded by attack of the ring onto an outside group already appended to the ring (a
Rings can be expanded by attack of the ring onto an outside group already appended to the ring (a
 A common method for expanding a ring involves opening
A common method for expanding a ring involves opening
 At bench scale, the reaction between cyclohexanone with
At bench scale, the reaction between cyclohexanone with 
 In the Arndt–Eistert reaction, an α-diazoketone is induced to release N2, resulting in a highly reactive sextet carbon center adjacent to the carbonyl. Such species convert by a Wolff rearrangement to give an ester in the presence of alcohols. When applied to cyclic α-diazoketones, ring contraction occurs. In the case of
In the Arndt–Eistert reaction, an α-diazoketone is induced to release N2, resulting in a highly reactive sextet carbon center adjacent to the carbonyl. Such species convert by a Wolff rearrangement to give an ester in the presence of alcohols. When applied to cyclic α-diazoketones, ring contraction occurs. In the case of
 An alternative to the standard Favorskii rearrangement, is to perform what can be thought of as a negative pinacol rearrangement where an anionic group encourages a bond aligned with a leaving group to migrate and expel the leaving group, which has been used in several syntheses. It should also be noted that the so-called "quasi-Favorskii rearrangement" proceeds without an additional nucleophile to form the final contracted product.
An alternative to the standard Favorskii rearrangement, is to perform what can be thought of as a negative pinacol rearrangement where an anionic group encourages a bond aligned with a leaving group to migrate and expel the leaving group, which has been used in several syntheses. It should also be noted that the so-called "quasi-Favorskii rearrangement" proceeds without an additional nucleophile to form the final contracted product.
 Ring expansion and contraction reactions are common for transition
Ring expansion and contraction reactions are common for transition
ring
(The) Ring(s) may refer to:
* Ring (jewellery), a round band, usually made of metal, worn as ornamental jewelry
* To make a sound with a bell, and the sound made by a bell
Arts, entertainment, and media Film and TV
* ''The Ring'' (franchise), a ...
s, usually in organic chemistry
Organic chemistry is a subdiscipline within chemistry involving the science, scientific study of the structure, properties, and reactions of organic compounds and organic matter, organic materials, i.e., matter in its various forms that contain ...
. The term usually refers to reactions involve making and breaking C-C bonds, Diverse pathways lead to these kinds of reactions. Many of these reactions are primarily of theoretical or pedagoogical interest, but some are very useful.

Ring expansions
 Rings can be expanded by attack of the ring onto an outside group already appended to the ring (a
Rings can be expanded by attack of the ring onto an outside group already appended to the ring (a migration
Migration, migratory, or migrate may refer to: Human migration
* Human migration, physical movement by humans from one region to another
** International migration, when peoples cross state boundaries and stay in the host state for some minimum le ...
/insertion), opening of a bicycle to a single larger ring, or coupling a ring closing with an expansion. These expansions can be further broken down by what type of atom they incorporate (a carbon or a heteroatom) into the expanded ring.
Carbon insertion through migration to an exocyclic group
These reactions have the general features of having an exocyclic leaving group on a carbon adjacent to the ring and an electron donating group on the ring capable of initiating a migration of an endocyclic bond. A common migration introduction of carbon is a pinacol rearrangement. While this reaction refers specifically to a vicinal dihydroxide rearrangement, there are other pinacol type rearrangements that proceed through the same general mechanism such as the Tiffeneau–Demjanov rearrangement. These "semipinacol rearrangements" occur under milder conditions and are thus preferable in complex syntheses. These reactions are useful beyond simply expanding a ring because the exocyclic group attacked may also have other functionality appended to it besides the leaving group. The group to which the endocyclic bond migrates can also be selectively added to the ring based on the functionality already present, for example 1,2 addition into a cyclic ketone.Carbon insertion through opening of a bicycle
 A common method for expanding a ring involves opening
A common method for expanding a ring involves opening cyclopropane
Cyclopropane is the cycloalkane with the molecular formula (CH2)3, consisting of three methylene groups (CH2) linked to each other to form a triangular ring. The small size of the ring creates substantial ring strain in the structure. Cyclopropane ...
-containing bicyclic intermediate. The strategy can start with a Simmons-Smith-like cyclopropanation of a cyclic alkene.
A related cyclopropane-based ring expansion is the Buchner ring expansion. The Buchner ring expansion is used to convert arenes to cycloheptatriene
Cycloheptatriene (CHT) is an organic compound with the chemical formula, formula C7H8. It is a closed ring of seven carbon atoms joined by three double bonds (as the name implies) and four single bonds. This colourless liquid has been of recurring ...
s. The Buchner ring expansion is encouraged to open to the desired product by placing electron withdrawing groups on the carbon added. In order to perform the ring opening on saturated bicyclic molecules the cyclopropane must be introduced such that a neighboring group can facilitate the expansion or the ring must be opened by attackate the expansion or the ring must be opened by attack from an outside group.
Ring opening as a means of ring expansion can also be applied to larger systems to give access to even larger ring syscyclization. The Grob fragmentation
A Grob fragmentation is an elimination reaction that breaks a neutral aliphatic chain into three fragments: a cation, positive ion spanning atoms 1 and 2 (the "electrofuge"), an unsaturated hydrocarbon, unsaturated neutral fragment spanning positio ...
can be applied as an example of such an expansion. Like the pinacol type migration the Grob fragmentation relies on an electron donating group to promote the bond migration and encourage the leaving group to be expelled. In this case the electron donating group can be a pseudo electron donating group which is capable of eliminating and donating an electron pair into the carbon with the breaking bond. Working with two smaller rings can allow for elaboration of two parts of the molecule separately before working with the expanded ring. The Dowd-Beckwith ring expansion reaction is also capable of adding several carbons to a ring at a time, and is a useful tool for making large rings. While it proceeds through an intermediate bicycle the final cyclization and ring opening take place within the same radical reaction
A free-radical reaction is any chemical reaction involving free radicals. This reaction type is abundant in organic reactions. Two pioneering studies into free radical reactions have been the discovery of the triphenylmethyl radical by Moses Gomb ...
. This expansion is useful because it allows the expansion of a beta-ketoester to a large cyclic ketone which can easily be elaborated using either the cyclic ketone or the exocyclic ester.
Heteroatom insertion reactions
Someheterocycle
A heterocyclic compound or ring structure is a cyclic compound that has atoms of at least two different elements as members of its ring(s). Heterocyclic organic chemistry is the branch of organic chemistry dealing with the synthesis, proper ...
s can be made through ring expansions. Beckmann rearrangement
World demand for caprolactam was estimated to reach five million tons per year for 2015. It has been estimated that 90% of all caprolactam is synthesised fromcyclohexanone
Cyclohexanone is the organic compound with the formula (CH2)5CO. The molecule consists of six-carbon cyclic molecule with a ketone functional group. This colorless oily liquid has a sweet odor reminiscent of benzaldehyde. Over time, samples of ...
(1), which is first converted to its oxime
In organic chemistry, an oxime is an organic compound belonging to the imines, with the general Chemical formula, formula , where R is an organic Side chain, side-chain and R' may be hydrogen, forming an aldoxime, or another organic functional g ...
. Treatment of this oxime with acid induces the Beckmann rearrangement
The Beckmann rearrangement, named after the German chemist Ernst Otto Beckmann (1853–1923), is a rearrangement reaction, rearrangement of an oxime functional group to substituted amides. The rearrangement has also been successfully performed on ...
to give caprolactam:
:
The Beckmann rearrangement has also been used for the introduction of nitrogen into codeine.Other N-insertions
A minor industrial route involves the treatment ofcyclohexanecarboxylic acid
Cyclohexanecarboxylic acid is the organic compound with the formula C6H11CO2H. It is the carboxylic acid of cyclohexane. It is a colorless oil that crystallizes near room temperature..
Preparation and reactions
It is prepared by hydrogenation of ...
with nitrosylsulfuric acid (the Snia Viscosa process). This is thought to proceed via a ketene
In organic chemistry, a ketene is an organic compound of the form , where R and R' are two arbitrary valence (chemistry), monovalent functional group, chemical groups (or two separate Substituent, substitution sites in the same molecule). The na ...
.
:hydrazoic acid
Hydrazoic acid, also known as hydrogen azide, azic acid or azoimide, This also contains a detailed description of the contemporaneous production process. is a compound with the chemical formula . It is a colorless, volatile, and explosive liquid ...
gives caprolactam in the Schmidt reaction.
:
Baeyer-Villiger oxidation
In the Baeyer-Villiger oxidation an O atom is introduced into a ring.Ring contractions
Ring contractions are useful for making smaller, more strained rings from larger rings. The impetus for making these rings comes from the difficulty associated with making a fully elaborated small ring when such a ring could more easily be made from an elaborated larger ring, from which an atom can be excised, or that the original larger scaffold is more accessible. Ring contractions proceed via anionic, cationic, and carbenoid intermediates.Carbenoid ring contractions
 In the Arndt–Eistert reaction, an α-diazoketone is induced to release N2, resulting in a highly reactive sextet carbon center adjacent to the carbonyl. Such species convert by a Wolff rearrangement to give an ester in the presence of alcohols. When applied to cyclic α-diazoketones, ring contraction occurs. In the case of
In the Arndt–Eistert reaction, an α-diazoketone is induced to release N2, resulting in a highly reactive sextet carbon center adjacent to the carbonyl. Such species convert by a Wolff rearrangement to give an ester in the presence of alcohols. When applied to cyclic α-diazoketones, ring contraction occurs. In the case of steroid
A steroid is an organic compound with four fused compound, fused rings (designated A, B, C, and D) arranged in a specific molecular configuration.
Steroids have two principal biological functions: as important components of cell membranes t ...
s, this reaction has been used to convert cyclopentanone groups to cyclobutanyl derivatives.
Favorskii rearrangement
TheFavorskii rearrangement
The Favorskii rearrangement is principally a rearrangement of cyclopropanones and α-halo ketones that leads to carboxylic acid derivatives. In the case of cyclic α-halo ketones, the Favorskii rearrangement constitutes a ring contraction. This ...
is a classic anionic ring contraction. It proceeds through a carbanion that attacks an endocyclic carbon and expels a leaving group (a halide) forming a bicyclic molecule with rings smaller than the original. The bicycle is then opened by nucleophilic attack on the ketone to give the contracted product. This reaction has been used to convert cyclohexanone
Cyclohexanone is the organic compound with the formula (CH2)5CO. The molecule consists of six-carbon cyclic molecule with a ketone functional group. This colorless oily liquid has a sweet odor reminiscent of benzaldehyde. Over time, samples of ...
to the methyl ester of cyclopentanecarboxylic acid
 An alternative to the standard Favorskii rearrangement, is to perform what can be thought of as a negative pinacol rearrangement where an anionic group encourages a bond aligned with a leaving group to migrate and expel the leaving group, which has been used in several syntheses. It should also be noted that the so-called "quasi-Favorskii rearrangement" proceeds without an additional nucleophile to form the final contracted product.
An alternative to the standard Favorskii rearrangement, is to perform what can be thought of as a negative pinacol rearrangement where an anionic group encourages a bond aligned with a leaving group to migrate and expel the leaving group, which has been used in several syntheses. It should also be noted that the so-called "quasi-Favorskii rearrangement" proceeds without an additional nucleophile to form the final contracted product.
Cation contractions
The cationic rearrangement contraction proceeds through the loss of a leaving group and the migration of an endocyclic bond to the carbocation. Pinacol type rearrangements are often used for this type of contraction. Like the expansion reaction this proceeds with an electron donating group aiding in the migration. Contraction reactions of one ring can be coupled with an expansion of another to give an unequal bicycle from equally sized fused ring. These cationic rearrangements have found use to synthesize the cores of complex molecules.Expansions and contractions
The Demyanov ring contraction and expansion entails diazotization of aminocyclobutanes and aminocyclopropanes. Loss of N2 from the diazo cations results in secondary carbocations, which tend to rearrange and then undergo hydrolysis. The reaction converts aminocyclobutane into a mixture of hydroxycyclobutane and hydroxymethylcyclopropane. These reactions produce an equilibrating mixture of twocarbocation
Carbocation is a general term for ions with a positively charged carbon atom. In the present-day definition given by the IUPAC, a carbocation is any even-electron cation with significant partial positive charge on a carbon atom. They are further ...
s:
:
Inorganic and organometallic examples
metal complex
A coordination complex is a chemical compound consisting of a central atom or ion, which is usually metallic and is called the ''coordination centre'', and a surrounding array of bound molecules or ions, that are in turn known as ''ligands'' or ...
es.
Further reading
* *Redmore, D.; Gutsche, C.D. Carbocyclic Ring Expansion Reactions, Academic Press, NY, 1968, *{{cite journal , doi=10.1021/ja00245a069 , title=A new tributyltin hydride-based rearrangement of bromomethyl .beta.-keto esters. A synthetically useful ring expansion to .gamma.-keto esters , date=1987 , last1=Dowd , first1=Paul , last2=Choi , first2=Soo Chang , journal=Journal of the American Chemical Society , volume=109 , issue=11 , pages=3493–3494 , bibcode=1987JAChS.109.3493DReferences