Rieche Formylation on:
[Wikipedia]
[Google]
[Amazon]
Rieche formylation is a type of formylation reaction. The substrates are electron rich 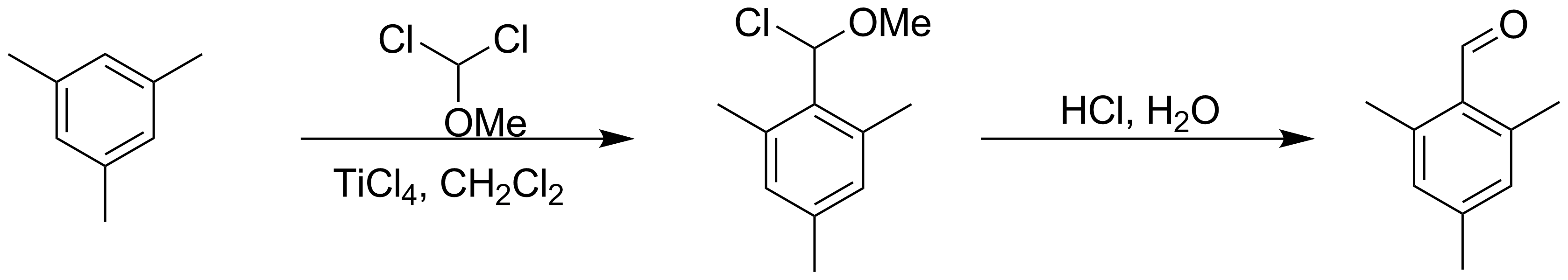
aromatic compound
Aromatic compounds, also known as "mono- and polycyclic aromatic hydrocarbons", are organic compounds containing one or more aromatic rings. The parent member of aromatic compounds is benzene. The word "aromatic" originates from the past grouping ...
s, such as mesitylene
Mesitylene or 1,3,5-trimethylbenzene is a derivative of benzene with three methyl substituents positioned symmetrically around the ring. The other two isomeric trimethylbenzenes are 1,2,4-trimethylbenzene (pseudocumene) and 1,2,3-trimethylbenze ...
or phenol
Phenol (also called carbolic acid) is an aromatic organic compound with the molecular formula . It is a white crystalline solid that is volatile. The molecule consists of a phenyl group () bonded to a hydroxy group (). Mildly acidic, it ...
s, with dichloromethyl methyl ether
Dichloromethyl methyl ether (HCl2COCH3) is an organic compound that belongs to the class of ethers with a dichloromethyl group and a methyl group. It can be synthesized from methyl formate and a mixture of phosphorus pentachloride and phosphorus o ...
acting as the formyl source. The catalyst
Catalysis () is the process of increasing the rate of a chemical reaction by adding a substance known as a catalyst (). Catalysts are not consumed in the reaction and remain unchanged after it. If the reaction is rapid and the catalyst recyc ...
is titanium tetrachloride
Titanium tetrachloride is the inorganic compound with the formula . It is an important intermediate in the production of titanium metal and the pigment titanium dioxide. is a volatile liquid. Upon contact with humid air, it forms thick clouds ...
and the workup is acidic. The reaction is named after Alfred Rieche who discovered it in 1960.

See also
Reimer–Tiemann reaction.References
{{Reflist Organic reactions Formylation reactions Carbon-carbon bond forming reactions Name reactions