Rhododendrol on:
[Wikipedia]
[Google]
[Amazon]
Rhododendrol (RD) also called 4- 3R)-3-hydroxybutylhenol (systemic name), is an
 In some individuals, a T-cell response is observed. The melanocyte cell lysates may sensitise T-cells, and the immunised
In some individuals, a T-cell response is observed. The melanocyte cell lysates may sensitise T-cells, and the immunised
organic compound
In chemistry, organic compounds are generally any chemical compounds that contain carbon- hydrogen or carbon-carbon bonds. Due to carbon's ability to catenate (form chains with other carbon atoms), millions of organic compounds are known. Th ...
with the formula C10H14O2. It is a naturally occurring ingredient present in many plants, such as the Rhododendron
''Rhododendron'' (; from Ancient Greek ''rhódon'' "rose" and ''déndron'' "tree") is a very large genus of about 1,024 species of woody plants in the heath family (Ericaceae). They can be either evergreen or deciduous. Most species are nativ ...
. The phenolic compound
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of one or more hydroxyl Functional group, groups (—Oxygen, OHydrogen, H) Chemical bond, bonded directly to an aromatic hydrocarbon group. The ...
was first developed in 2010 as a tyrosinase
Tyrosinase is an oxidase that is the rate-limiting enzyme for controlling the production of melanin. The enzyme is mainly involved in two distinct reactions of melanin synthesis otherwise known as the Raper Mason pathway. Firstly, the hydroxyl ...
inhibitor for skin-lightening cosmetics. In 2013, after rhododendrol reportedly caused skin depigmentation in consumers using RD-containing skin-brightening cosmetics, the cosmetics were withdrawn from the market. The skin condition, caused by RD, is called RD-induced leukoderma
Vitiligo is a disorder that causes the skin to lose its color. Specific causes are unknown but studies suggest a link to immune system changes.
Signs and symptoms
The only sign of vitiligo is the presence of pale patchy areas of depigmen ...
. Rhododendrol exerts melanocyte
Melanocytes are melanin-producing neural crest-derived cells located in the bottom layer (the stratum basale) of the skin's epidermis, the middle layer of the eye (the uvea),
the inner ear,
vaginal epithelium, meninges,
bones,
and hear ...
cytotoxicity via a tyrosinase-dependent mechanism. It has been shown to impair the normal proliferation of melanocytes through reactive oxygen species
In chemistry, reactive oxygen species (ROS) are highly reactive chemicals formed from diatomic oxygen (). Examples of ROS include peroxides, superoxide, hydroxyl radical, singlet oxygen, and alpha-oxygen.
The reduction of molecular oxygen ...
-dependent activation of GADD45. It is now well established that rhododendrol is a potent tyrosinase
Tyrosinase is an oxidase that is the rate-limiting enzyme for controlling the production of melanin. The enzyme is mainly involved in two distinct reactions of melanin synthesis otherwise known as the Raper Mason pathway. Firstly, the hydroxyl ...
inhibitor.
Structure and synthesis
Structure
Rhododendrol occurs as the glucoside rhododendrin in leaves of the Rhododendron ( Ericacae), and it naturally occurs as a phenolic compound in plants such as ''Acer nikoense
''Acer maximowiczianum'' (Nikko maple; syn. ''A. nikoense'' Maxim.), is a species of maple widely distributed in China (Anhui, Hubei, Hunan, Jiangxi, Sichuan, Zhejiang) and Japan (Honshū, Kyūshū, Shikoku).Xu, T.-z., Chen, Y., de Jong, P. C. ...
'' , ''Betula platyphylla
''Betula platyphylla'', the Asian white birch or Japanese white birch, is a tree species in the family Betulaceae. It can be found in subarctic and temperate Asia in Japan, China, Korea, Mongolia, and Russian Far East and Siberia
Siberia ...
,'' and the Chinese red birch ''Betula Alba''. The compound can be obtained from alkylation of phenols (C6H5OH). The molecule has a ''para''-substituted structure, and one chiral center. Also, the compound has a natural charge.
Biosynthesis
There are several ways to synthesise rhododendrol. First, the synthesis can be achieved in six steps frombenzaldehyde
Benzaldehyde (C6H5CHO) is an organic compound consisting of a benzene ring with a formyl substituent. It is the simplest aromatic aldehyde and one of the most industrially useful.
It is a colorless liquid with a characteristic almond-like odor. ...
. The key reactions in this method include aldol condensation and trichloroacetimidate glycosylation. The compound can also be prepared by reducing raspberry ketone
Raspberry ketone is a natural phenolic compound that is the primary aroma compound of red raspberries.
Occurrence
Raspberry ketone occurs in a variety of fruits, including raspberries, cranberries, and blackberries. It is biosynthesized from c ...
(4-(4-hydroxyphenyl)-2- butanone) with Raney nickel in EtOH. In addition, Rhododendrol can be synthesised from ''p''-coumaric acid. This pathway involves reduction of the aliphatic double bond present in ''p''-coumaric acid.
Mechanisms of action
The mechanism of action of rhododendrol has been investigated in multiple studies which revealed that RD competes withtyrosine
-Tyrosine or tyrosine (symbol Tyr or Y) or 4-hydroxyphenylalanine is one of the 20 standard amino acids that are used by cells to synthesize proteins. It is a non-essential amino acid with a polar side group. The word "tyrosine" is from the Gr ...
for hydroxylation by tyrosinase and interferes with melanin synthesis
Melanin (; from el, μέλας, melas, black, dark) is a broad term for a group of natural pigments found in most organisms. Eumelanin is produced through a multistage chemical process known as melanogenesis, where the oxidation of the amino ...
. First, RD is catalysed by tyrosinase to produce toxic metabolites as RD-cyclic catechol
Catechol ( or ), also known as pyrocatechol or 1,2-dihydroxybenzene, is a toxic organic compound with the molecular formula . It is the ''ortho'' isomer of the three isomeric benzenediols. This colorless compound occurs naturally in trace amoun ...
. These reactive metabolites cause damage to the melanocytes. There is still uncertainty, however, how the metabolites result in melanocyte damage.
A previous report reported that the melanocyte toxicity of rhododendrol is caused by the production of cytotoxic reactive oxygen species (ROS). However, another study stated that there was no ROS detected in the rhododendrol-treated melanocytes, but a tyrosinase-dependent accumulation of endoplasmic reticulum
The endoplasmic reticulum (ER) is, in essence, the transportation system of the eukaryotic cell, and has many other important functions such as protein folding. It is a type of organelle made up of two subunits – rough endoplasmic reticulum ( ...
stress and activation of the apoptotic pathway
Apoptosis (from grc, ἀπόπτωσις, apóptōsis, 'falling off') is a form of programmed cell death that occurs in multicellular organisms. Biochemical events lead to characteristic cell changes (morphology) and death. These changes includ ...
. Even though there is still no full agreement on the exact mechanism of action, it is suggested that the mechanism of RD-induced leukoderma
Vitiligo is a disorder that causes the skin to lose its color. Specific causes are unknown but studies suggest a link to immune system changes.
Signs and symptoms
The only sign of vitiligo is the presence of pale patchy areas of depigmen ...
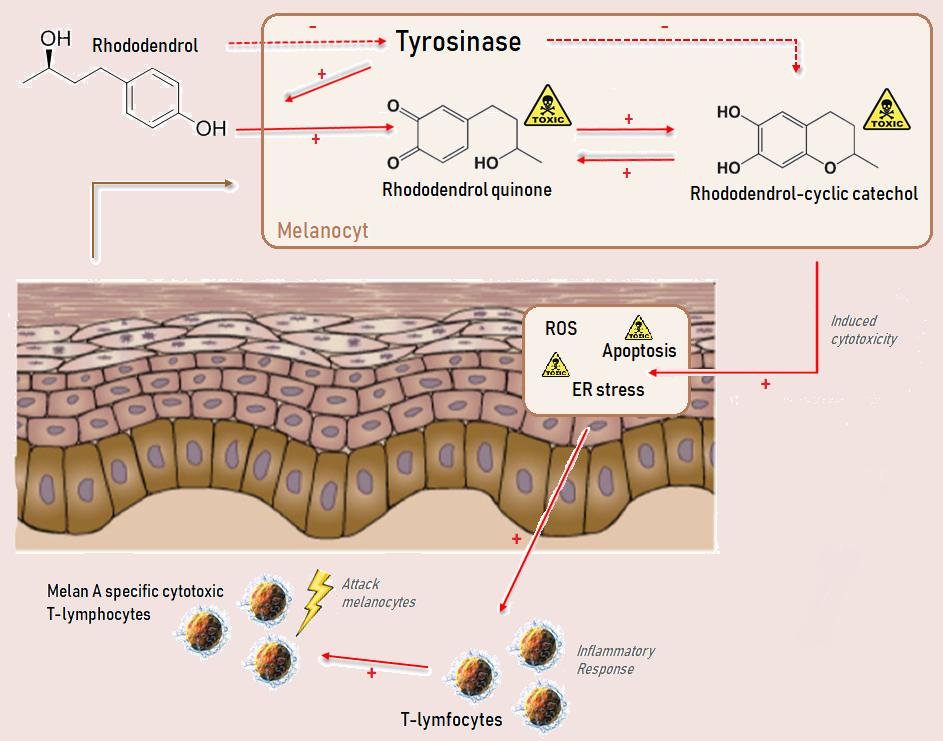
closely resembles the mechanism displayed in the figure below ( Suggested mechanism of Rhododendrol.png).
 In some individuals, a T-cell response is observed. The melanocyte cell lysates may sensitise T-cells, and the immunised
In some individuals, a T-cell response is observed. The melanocyte cell lysates may sensitise T-cells, and the immunised cytotoxic T-lymphocytes
A cytotoxic T cell (also known as TC, cytotoxic T lymphocyte, CTL, T-killer cell, cytolytic T cell, CD8+ T-cell or killer T cell) is a T lymphocyte (a type of white blood cell) that kills cancer cells, cells that are infected by intracellular pa ...
(specific to Melan A, which is a melanocytic differentiation marker
The term Marker may refer to:
Common uses
* Marker (linguistics), a morpheme that indicates some grammatical function
* Marker (telecommunications), a special-purpose computer
* Boundary marker, an object that identifies a land boundary
* Marke ...
) may enhance the RD-induced leukoderma or evoke vitiligo-like lesions on the non-applied skin.
Metabolism
Rhododendrol is metabolised via tyrosinase-catalysed oxidation. Therefore, the enzymetyrosinase
Tyrosinase is an oxidase that is the rate-limiting enzyme for controlling the production of melanin. The enzyme is mainly involved in two distinct reactions of melanin synthesis otherwise known as the Raper Mason pathway. Firstly, the hydroxyl ...
is necessary for the oxidation of rhododendrol. Tyrosinase regularly plays an essential role in the production of melanocytes called the melanogenesis. After oxidation of rhododendrol by the tyrosinase enzyme, several kinds of phenols
In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of one or more hydroxyl groups (— O H) bonded directly to an aromatic hydrocarbon group. The simplest is phenol, . Phenolic compounds ...
and catechol
Catechol ( or ), also known as pyrocatechol or 1,2-dihydroxybenzene, is a toxic organic compound with the molecular formula . It is the ''ortho'' isomer of the three isomeric benzenediols. This colorless compound occurs naturally in trace amoun ...
s are formed. These phenols and catechols together form ortho-quinones (o-quinones). Presence of o-quinones can lead to cytotoxicity via the production of reactive oxygen species
In chemistry, reactive oxygen species (ROS) are highly reactive chemicals formed from diatomic oxygen (). Examples of ROS include peroxides, superoxide, hydroxyl radical, singlet oxygen, and alpha-oxygen.
The reduction of molecular oxygen ...
(ROS) or by the binding to enzymes or DNA.
When rhododendrol is metabolised via the tyrosinase-catalysed oxidation RD-quinone will be formed. This formation gives rise to the formation of secondary quinones. As described in the mechanisms of action, the presence of quinones could cause cytotoxicity to melanocytes by the production of ROS or by binding to DNA and enzymes.
Adverse effects
Considering the use of rhododendrol is prohibited since 2013, the knowledge about the side effects rhodendodrol causes is limited. As stated above, the main known adverse effect of rhododendrol is melanocyte toxicity.Melanocyte
Melanocytes are melanin-producing neural crest-derived cells located in the bottom layer (the stratum basale) of the skin's epidermis, the middle layer of the eye (the uvea),
the inner ear,
vaginal epithelium, meninges,
bones,
and hear ...
s are melanin-producing cells, primarily responsible for skin colour. Melanocyte toxicity induces apoptosis of the cell, causing the melanocytes to die. This is due to an increased expression of caspase-3 and caspase-8. Caspase proteins are crucial mediators of apoptosis, with caspase-3 and caspase-8 being death proteases. Considering melanocytes are responsible for skin colour, apoptosis of these cells causes the colour of the skin to vanish. This disease caused by rhododendrol is called leukoderma
Vitiligo is a disorder that causes the skin to lose its color. Specific causes are unknown but studies suggest a link to immune system changes.
Signs and symptoms
The only sign of vitiligo is the presence of pale patchy areas of depigmen ...
. Leukoderma, also known as vitiligo, is a skin disease characterized by patches of the skin losing their pigment. This rhododendrol-induced depigmentation can be either long-term and short term. In most cases, repigmentation and cessation of further depigmentation occur after discontinuing the exposure to the substance. However, some patients develop vitiligo vulgaris through the spread of depigmentation into non-exposed areas. This only occurs after severe chemical damage. In addition, rhododendrol not only causes melanocytes to go into apoptosis but it also inhibits melanogenesis. Meaning that the use of rhododendrol not only causes melanocytes to die, but also prevents the development of new melanocytes.
Toxicity
Various studies have shown that there is more than one mechanism by which rhododendrol can have a toxic effect. This toxic effect of rhododendrol is found in the melanocytes, which gives rise to skin depigmentation.ROS
Rhododendrol can have a toxic effect via the production ofreactive oxygen species (ROS)
In chemistry, reactive oxygen species (ROS) are highly reactive chemicals formed from diatomic oxygen (). Examples of ROS include peroxides, superoxide, hydroxyl radical, singlet oxygen, and alpha-oxygen.
The reduction of molecular oxygen () ...
. This will cause an impairment in the further development of melanocytes in the skin. Impairment is caused by upregulation of the GADD45 gene. A study of Kim et al. showed that the production of ROS, which gives rise to more production of GADD45, is already found at low concentrations of rhododendrol. At the time that rhododendrol was used in cosmetic products, it contained concentrations of 2%. The study of Kim et al. suggests that the production of reactive oxygen species at low concentrations may have contributed to the development of leukoderma in users of these cosmetic products.
Reactive metabolites
The study of Ito et al. showed that rhododendrol exerts its toxic effect in the melanocytes via tyrosinase-dependent mechanisms. This tyrosinase enzyme breaks rhododendrol down into the following reactive metabolites: RD-quinone and RD-cyclic quinone. These reactive metabolites can bind to proteins which contain athiol
In organic chemistry, a thiol (; ), or thiol derivative, is any organosulfur compound of the form , where R represents an alkyl or other organic substituent. The functional group itself is referred to as either a thiol group or a sulfhydryl gro ...
-group or it can form radicals. These radicals are toxic to the melanocytes as it causes auto-oxidation of the cells. Auto-oxidation, in turn, causes oxidative stress to cells, which will impair the natural growth and function of the melanocytes.
Rhododenol and raspberry ketone impair the regular proliferation of melanocytes through reactive oxygen species-dependent activation of GADD45.
Effects on animals
The effect of rhododendrol (4-(4-hydroxyphenyl)-2-butanol) is measured in mice as well as in guinea pigs.{{Cite journal, last1=Kuroda, first1=Yasutaka, last2=Takahashi, first2=Yutaka, last3=Sakaguchi, first3=Hitoshi, last4=Matsunaga, first4=Kayoko, last5=Suzuki, first5=Tamio, date=2014, title=Depigmentation of the skin induced by 4-(4-hydroxyphenyl)-2-butanol is spontaneously re-pigmented in brown and black guinea pigs, journal=The Journal of Toxicological Sciences, volume=39, issue=4, pages=615–623, doi=10.2131/jts.39.615, pmid=25056786, issn=0388-1350, doi-access=free These studies were performed to elucidate the aetiology of RD-induced leukoderma. The data of these studies revealed that the amount of RD applied to the skin is highly relevant considering that high doses of RD are required in order to cause cytotoxicity. This finding is contrary to the results presented in the study of Kim et al., which is performed in humans. Furthermore, the animal studies enlightened the importance of the ER-stress response. It is suggested that the activity of the ER-stress response may determine whether melanocytes survive or die. Also, the study of Abe et al. revealed that theautophagy
Autophagy (or autophagocytosis; from the Ancient Greek , , meaning "self-devouring" and , , meaning "hollow") is the natural, conserved degradation of the cell that removes unnecessary or dysfunctional components through a lysosome-dependent re ...
pathway may be involved in the resistance to the cytotoxicity of RD.
Since the biochemical and histological characteristics of the used mice in the animal studies (hairless hk14-SCF Tg mice) closely resembled the characteristics of the human skin, these newly generated mice could be used as experimental animal models to investigate chemical vitiligo further.
References