Reversible addition−fragmentation chain-transfer polymerization on:
[Wikipedia]
[Google]
[Amazon]
 Reversible addition−fragmentation chain-transfer or RAFT polymerization is one of several kinds of
Reversible addition−fragmentation chain-transfer or RAFT polymerization is one of several kinds of
 RAFT is a type of
RAFT is a type of
 Enz-RAFT is a RAFT polymerization technique which allows for controlled oxygen-sensitive polymerization in an open vessel. Enz-RAFT uses 1–4 μM
Enz-RAFT is a RAFT polymerization technique which allows for controlled oxygen-sensitive polymerization in an open vessel. Enz-RAFT uses 1–4 μM
 Using a compound with multiple dithio moieties (often termed a multifunctional RAFT agent) can result in the formation of star, brush and comb polymers. Taking star polymers as an example, RAFT differs from other forms of living radical polymerization techniques in that either the R- or Z-group may form the core of the star (See Figure 7). While utilizing the R-group as the core results in similar structures found using ATRP or NMP, the ability to use the Z-group as the core makes RAFT unique. When the Z-group is used, the reactive polymeric arms are detached from the star's core during growth and to undergo chain transfer, must once again react at the core.
Using a compound with multiple dithio moieties (often termed a multifunctional RAFT agent) can result in the formation of star, brush and comb polymers. Taking star polymers as an example, RAFT differs from other forms of living radical polymerization techniques in that either the R- or Z-group may form the core of the star (See Figure 7). While utilizing the R-group as the core results in similar structures found using ATRP or NMP, the ability to use the Z-group as the core makes RAFT unique. When the Z-group is used, the reactive polymeric arms are detached from the star's core during growth and to undergo chain transfer, must once again react at the core.
 Reversible addition−fragmentation chain-transfer or RAFT polymerization is one of several kinds of
Reversible addition−fragmentation chain-transfer or RAFT polymerization is one of several kinds of reversible-deactivation radical polymerization
Reversible deactivation radical polymerizations (RDRPs) are members of the class of reversible deactivation polymerizations which exhibit much of the character of living polymerizations, but cannot be categorized as such as they are not without cha ...
. It makes use of a chain-transfer agent in the form of a thiocarbonylthio compound (or similar, from here on referred to as a RAFT agent, see Figure 1) to afford control over the generated molecular weight and polydispersity during a free-radical polymerization. Discovered at the Commonwealth Scientific and Industrial Research Organisation
The Commonwealth Scientific and Industrial Research Organisation (CSIRO) is an Australian Government agency responsible for scientific research.
CSIRO works with leading organisations around the world. From its headquarters in Canberra, CSIRO ...
(CSIRO) of Australia in 1998, RAFT polymerization is one of several living or controlled radical
Radical may refer to:
Politics and ideology Politics
* Radical politics, the political intent of fundamental societal change
*Radicalism (historical), the Radical Movement that began in late 18th century Britain and spread to continental Europe an ...
polymerization techniques, others being atom transfer radical polymerization Atom transfer radical polymerization (ATRP) is an example of a reversible-deactivation radical polymerization. Like its counterpart, ATRA, or atom transfer radical addition, ATRP is a means of forming a carbon-carbon bond with a transition metal ...
(ATRP) and nitroxide-mediated polymerization (NMP), etc. RAFT polymerization uses thiocarbonylthio compounds, such as dithioesters, thiocarbamates, and xanthate
150px, Sodium salt of ethyl xanthate
Xanthate usually refers to a salt with the formula (R = alkyl; M+ = Na+, K+), thus they are the metal-thioate/''O''-esters of dithiocarbonate. The name ''xanthates'' is derived from Ancient Greek ''xanthos' ...
s, to mediate the polymerization via a reversible chain-transfer process. As with other controlled radical polymerization techniques, RAFT polymerizations can be performed with conditions to favor low dispersity (molecular weight distribution) and a pre-chosen molecular weight. RAFT polymerization can be used to design polymers of complex architectures, such as linear block copolymers, comb-like, star, brush polymers, dendrimer
Dendrimers are highly ordered, branched polymeric molecules. Synonymous terms for dendrimer include arborols and cascade molecules. Typically, dendrimers are symmetric about the core, and often adopt a spherical three-dimensional morphology. The ...
s and cross-linked networks.
Overview
History
The addition−fragmentation chain-transfer process was first reported in the early 1970s. However, the technique was irreversible, so the transfer reagents could not be used to control radical polymerization at this time. For the first few years addition−fragmentation chain-transfer was used to help synthesize end-functionalized polymers. Scientists began to realize the potential of RAFT in controlled radical polymerization in the 1980s.Macromonomers
A macromonomer is a macromolecule with one end-group that enables it to act as a monomer. Macromonomers will contribute a single monomeric unit to a chain of the completed macromolecule.
Several macromonomers have been successfully synthesized ut ...
were known as reversible chain transfer agents during this time, but had limited applications on controlled radical polymerization.
In 1995, a key step in the "degenerate" reversible chain transfer step for chain equilibration was brought to attention. The essential feature is that the product of chain transfer is also a chain transfer agent with similar activity to the precursor transfer agent.
RAFT polymerization today is mainly carried out by thiocarbonylthio chain transfer agents. It was first reported by Rizzardo ''et al.'' in 1998. RAFT is one of the most versatile methods of controlled radical polymerization because it is tolerant of a very wide range of functionality in the monomer and solvent, including aqueous solutions. RAFT polymerization has also been effectively carried out over a wide temperature range.
Important components of RAFT
Typically, a RAFT polymerization system consists of: * a radical source (e.g. thermochemical initiator or the interaction of gamma radiation with some reagent) *monomer
In chemistry, a monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.
Classification
...
* RAFT agent
* solvent (not strictly required if the monomer is a liquid)
A temperature is chosen such that (a) chain growth occurs at an appropriate rate, (b) the chemical initiator (radical source) delivers radicals at an appropriate rate and (c) the central RAFT equilibrium (see later) favors the active rather than dormant state to an acceptable extent.
RAFT polymerization can be performed by adding a chosen quantity of an appropriate RAFT agent to a conventional free radical polymerization. Usually the same monomers, initiators, solvents and temperatures can be used.
Radical initiators such as azobisisobutyronitrile
Azobisisobutyronitrile (abbreviated AIBN) is an organic compound with the formula CH3)2C(CN)sub>2N2. This white powder is soluble in alcohols and common organic solvents but is insoluble in water. It is often used as a foamer in plastics and rub ...
(AIBN) and 4,4'-azobis(4-cyanovaleric acid) (ACVA), also called 4,4'-azobis(4-cyanopentanoic acid)
4,4′-Azobis(4-cyanopentanoic acid) (ACPA) is a free radical initiator used in polymer synthesis. ACPA is a water-soluble initiator used in both heterogeneous and homogeneous free-radical polymerizations. It is used as an initiator in reversibl ...
, are widely used as the initiator in RAFT.
Figure 3 provides a visual description of RAFT polymerizations of poly(methyl methacrylate)
Poly(methyl methacrylate) (PMMA) belongs to a group of materials called engineering plastics. It is a transparent thermoplastic. PMMA is also known as acrylic, acrylic glass, as well as by the trade names and brands Crylux, Plexiglas, Acryli ...
and polyacrylic acid
Poly(acrylic acid) (PAA; trade name Carbomer) is a polymer with the formula (CH2-CHCO2H)n. It is a derivative of acrylic acid (CH2=CHCO2H). In addition to the homopolymers, a variety of copolymers and crosslinked polymers, and partially deproto ...
using AIBN as the initiator and two RAFT agents.
RAFT polymerization is known for its compatibility with a wide range of monomers compared to other controlled radical polymerization
Living free radical polymerization is a type of living polymerization where the active polymer chain end is a free radical. Several methods exist. IUPAC recommends to use the term " reversible-deactivation radical polymerization" instead of "liv ...
s. These monomers include (meth)acrylates, (meth)acrylamides, acrylonitrile, styrene and derivatives, butadiene, vinyl acetate and N-vinylpyrrolidone. The process is also suitable for use under a wide range of reaction parameters such as temperature or the level of impurities, as compared to NMP or ATRP.
The Z and R group of a RAFT agent must be chosen according to a number of considerations. The Z group primarily affects the stability of the S=C bond and the stability of the adduct radical (Polymer-S-C•(Z)-S-Polymer, see section on Mechanism). These in turn affect the position of and rates of the elementary reactions in the pre- and main-equilibrium. The R group must be able to stabilize a radical such that the right hand side of the pre-equilibrium is favored, but unstable enough that it can reinitiate growth of a new polymer chain. As such, a RAFT agent must be designed with consideration of the monomer and temperature, since both these parameters also strongly influence the kinetics and thermodynamics of the RAFT equilibria.
Products
The desired product of a RAFT polymerization is typically linear polymer with an R-group at one end and a dithiocarbonate moiety at the other end. Figure 4 depicts the major and minor products of a RAFT polymerization. All other products arise from (a) biradical termination events or (b) reactions of chemical species that originate from initiator fragments, denoted by I in the figure. (Note that categories (a) and (b) intersect). The selectivity towards the desired product can be increased by increasing the concentration of RAFT agent relative to the quantity of free radicals delivered during the polymerization. This can be done either directly (i.e. by increasing the RAFT agent concentration) or by decreasing the rate of decomposition of or concentration of initiator.RAFT mechanism
Kinetics overview
 RAFT is a type of
RAFT is a type of living polymerization
In polymer chemistry, living polymerization is a form of chain growth polymerization where the ability of a growing polymer chain to terminate has been removed. This can be accomplished in a variety of ways. Chain termination and chain transfer ...
involving a conventional radical polymerization
In polymer chemistry, free-radical polymerization (FRP) is a method of polymerization by which a polymer forms by the successive addition of free-radical building blocks ( repeat units). Free radicals can be formed by a number of different mechan ...
which is mediated by a RAFT agent. Monomers must be capable of radical polymerization. There are a number of steps in a RAFT polymerization: initiation, pre-equilibrium, re-initiation, main equilibrium, propagation and termination.
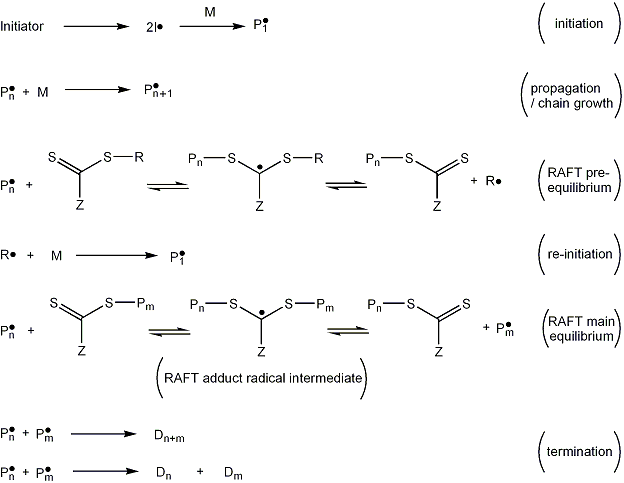
The mechanism is now explained further with the help of Figure 5.
Initiation: The reaction is started by a free-radical source which may be a decomposing radical initiator
In chemistry, radical initiators are substances that can produce radical species under mild conditions and promote radical reactions. These substances generally possess weak bonds—bonds that have small bond dissociation energies. Radical i ...
such as AIBN. In the example in Figure 5, the initiator decomposes to form two fragments (I•) which react with a single monomer molecule to yield a propagating (i.e. growing) polymeric radical of length 1, denoted P1•.
Propagation: Propagating radical chains of length ''n'' in their active (radical) form, Pn•, add to monomer, M, to form longer propagating radicals, Pn+1•.
RAFT pre-equilibrium: A polymeric radical with n monomer units (Pn) reacts with the RAFT agent to form a RAFT adduct radical. This may undergo a fragmentation reaction in either direction to yield either the starting species or a radical (R•) and a polymeric RAFT agent (S=C(Z)S-Pn). This is a reversible step in which the intermediate RAFT adduct radical is capable of losing either the R group (R•) or the polymeric species (Pn•).
Re-initiation: The leaving group radical (R•) then reacts with another monomer species, starting another active polymer chain.
Main RAFT equilibrium: This is the most important part in the RAFT process, in which, by a process of rapid interchange, the present radicals (and hence opportunities for polymer chain growth) are "shared" among all species that have not yet undergone termination (Pn• and S=C(Z)S-Pn). Ideally the radicals are shared equally, causing chains to have equal opportunities for growth and a narrow PDI.
Termination: Chains in their active form react via a process known as bi-radical termination to form chains that cannot react further, known as dead polymer. Ideally, the RAFT adduct radical is sufficiently hindered such that it does not undergo termination reactions.
Thermodynamics of the main RAFT equilibrium
The position of the main RAFT equilibrium (Figure 5) is affected by the relative stabilities of the RAFT adduct radical (Pn-S-C•(Z)-S-Pm) and its fragmentation products, namely S=C(Z)S-Pn and polymeric radical (Pm•). If formation of the RAFT adduct radical is sufficiently thermodynamically favorable, the concentration of active species, Pm•, will be reduced to the extent that a reduction in the rate of conversion of monomer into polymer is also observed, as compared to an equivalent polymerization without RAFT agent. Such a polymerization, is referred to as a rate-retarded RAFT polymerization. The rate of a RAFT polymerization, that is, the rate of conversion of monomer into polymer, mainly depends on the rate of the Propagation reaction (Figure 5) because the rate of initiation and termination are much higher than the rate of propagation. The rate of propagation is proportional to the concentration,•
In typography, a bullet or bullet point, , is a typographical symbol or glyph used to introduce items in a list. For example:
*Point 1
*Point 2
*Point 3
The bullet symbol may take any of a variety of shapes, such as circular, square, diamo ...
of the active species P•, whereas the rate of the termination reaction, being second order, is proportional to the square •
In typography, a bullet or bullet point, , is a typographical symbol or glyph used to introduce items in a list. For example:
*Point 1
*Point 2
*Point 3
The bullet symbol may take any of a variety of shapes, such as circular, square, diamo ...
sup>2. This means that during rate-retarded RAFT polymerizations, the rate of formation of termination products is suppressed to a greater extent than the rate of chain growth.
In RAFT polymerizations without rate-retardation, the concentration of the active species P• is close to that in an equivalent conventional polymerization in the absence of RAFT agent.
The main RAFT equilibrium and hence the rate retardation of the reaction is influenced by both temperature and chemical factors. A high temperature favors formation of the fragmentation products rather than the adduct radical Pn-S-C•(Z)-S-Pm. RAFT agents with a radical stabilising Z-group such as Phenyl group
In organic chemistry, the phenyl group, or phenyl ring, is a cyclic group of atoms with the formula C6 H5, and is often represented by the symbol Ph. Phenyl group is closely related to benzene and can be viewed as a benzene ring, minus a hydroge ...
favor the adduct radical, as do propagating radicals whose monomers lack radical stabilising features, for example Vinyl acetate
Vinyl acetate is an organic compound with the formula CH3CO2CH=CH2. This colorless liquid is the precursor to polyvinyl acetate and ethene-vinyl acetate copolymers, important industrial polymers.
Production
The worldwide production capacity of ...
.
Further mechanistic considerations
In terms of mechanism, an ideal RAFT polymerization has several features. The pre-equilibrium and re-initiation steps are completed very early in the polymerization meaning that the major product of the reaction (the RAFT polymer chains, RAFT-Pn), all start growing at approximately the same time. The forward and reverse reactions of the main RAFT equilibrium are fast, favoring equal growth opportunities amongst the chains. The total number of radicals delivered to the system by the initiator during the course of the polymerization is low compared to the number of RAFT agent molecules, meaning that the R group initiated polymer chains from the re-initiation step form the majority of the chains in the system, rather than initiator fragment bearing chains formed in the Initiation step. This is important because initiator decomposes continuously during the polymerization, not just at the start, and polymer chains arising from initiator decomposition cannot, therefore, have a narrow length distribution. These mechanistic features lead to an average chain length that increases linearly with the conversion of monomer into polymer. In contrast to other controlled radical polymerizations (for example ATRP), a RAFT polymerization does not achieve controlled evolution of molecular weight and low polydispersity by reducing bi-radical termination events (although in some systems, these events may indeed be reduced somewhat, as outlined above), but rather, by ensuring that most polymer chains start growing at approximately the same time and experience equal growth during polymerization.Enz-RAFT
 Enz-RAFT is a RAFT polymerization technique which allows for controlled oxygen-sensitive polymerization in an open vessel. Enz-RAFT uses 1–4 μM
Enz-RAFT is a RAFT polymerization technique which allows for controlled oxygen-sensitive polymerization in an open vessel. Enz-RAFT uses 1–4 μM glucose oxidase
The glucose oxidase enzyme (GOx or GOD) also known as notatin (EC number 1.1.3.4) is an oxidoreductase that catalyses the oxidation of glucose to hydrogen peroxide and D-glucono-δ-lactone. This enzyme is produced by certain species of fungi and ...
to remove dissolved oxygen from the system. As the degassing
Degassing, also known as degasification, is the removal of dissolved gases from liquids, especially water or aqueous solutions. There are numerous methods for removing gases from liquids.
Gases are removed for various reasons. Chemists remove ga ...
is decoupled from the polymerization, initiator concentrations can be reduced, allowing for high control and end group fidelity. Enz-RAFT can be used in a number of organic solvent systems, with high activity in up to 80% tert-butanol
''tert''-Butyl alcohol is the simplest tertiary alcohol, with a formula of (CH3)3COH (sometimes represented as ''t''-BuOH). Its isomers are 1-butanol, isobutanol, and butan-2-ol. ''tert''-Butyl alcohol is a colorless solid, which melts near ro ...
, acetonitrile, and dioxane
1,4-Dioxane () is a heterocyclic organic compound, classified as an ether. It is a colorless liquid with a faint sweet odor similar to that of diethyl ether. The compound is often called simply dioxane because the other dioxane isomers ( 1,2- ...
. With Enz-RAFT, polymerizations do not require prior degassing making this technique convenient for the preparation of most polymers by RAFT. The technique was developed at Imperial College London
Imperial College London (legally Imperial College of Science, Technology and Medicine) is a public research university in London, United Kingdom. Its history began with Prince Albert, consort of Queen Victoria, who developed his vision for a ...
by Robert Chapman and Adam Gormley in the lab of Molly Stevens
Molly Morag Stevens is Professor of Biomedical Materials and regenerative medicine and Research Director for Biomedical Materials Sciences in the Institute of Biomedical Engineering at Imperial College London.
.
Applications
RAFT polymerization has been used to synthesize a wide range of polymers with controlled molecular weight and low polydispersities (between 1.05 and 1.4 for many monomers). RAFT polymerization is known for its compatibility with a wide range of monomers as compared to othercontrolled radical polymerization
Living free radical polymerization is a type of living polymerization where the active polymer chain end is a free radical. Several methods exist. IUPAC recommends to use the term " reversible-deactivation radical polymerization" instead of "liv ...
s. Some monomers
In chemistry, a monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.
Classification
...
capable of polymerizing by RAFT include styrenes, acrylates, acrylamides, and many vinyl monomers. Additionally, the RAFT process allows the synthesis of polymers with specific macromolecular architectures such as block, gradient
In vector calculus, the gradient of a scalar-valued differentiable function of several variables is the vector field (or vector-valued function) \nabla f whose value at a point p is the "direction and rate of fastest increase". If the gr ...
, statistical, comb, brush, star, hyperbranched, and network copolymers
In polymer chemistry, a copolymer is a polymer derived from more than one species of monomer. The polymerization of monomers into copolymers is called copolymerization. Copolymers obtained from the copolymerization of two monomer species are so ...
. These properties make RAFT useful in many types of polymer synthesis.
Block copolymers
As with other living radical polymerization techniques, RAFT allows chain extension of a polymer of one monomer with a second type of polymer to yield a block copolymer. In such a polymerisation, there is the additional challenge that the RAFT agent for the first monomer must also be suitable for the second monomer, making block copolymerisation of monomers of highly disparate character challenging. Multiblock copolymers have also been reported by using difunctional R groups or symmetrical trithiocarbonates with difunctional Z groups.Star, brush and comb polymers
 Using a compound with multiple dithio moieties (often termed a multifunctional RAFT agent) can result in the formation of star, brush and comb polymers. Taking star polymers as an example, RAFT differs from other forms of living radical polymerization techniques in that either the R- or Z-group may form the core of the star (See Figure 7). While utilizing the R-group as the core results in similar structures found using ATRP or NMP, the ability to use the Z-group as the core makes RAFT unique. When the Z-group is used, the reactive polymeric arms are detached from the star's core during growth and to undergo chain transfer, must once again react at the core.
Using a compound with multiple dithio moieties (often termed a multifunctional RAFT agent) can result in the formation of star, brush and comb polymers. Taking star polymers as an example, RAFT differs from other forms of living radical polymerization techniques in that either the R- or Z-group may form the core of the star (See Figure 7). While utilizing the R-group as the core results in similar structures found using ATRP or NMP, the ability to use the Z-group as the core makes RAFT unique. When the Z-group is used, the reactive polymeric arms are detached from the star's core during growth and to undergo chain transfer, must once again react at the core.
Smart materials and biological applications
Due to its flexibility with respect to the choice ofmonomer
In chemistry, a monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.
Classification
...
s and reaction conditions, the RAFT process competes favorably with other forms of living polymerization
In polymer chemistry, living polymerization is a form of chain growth polymerization where the ability of a growing polymer chain to terminate has been removed. This can be accomplished in a variety of ways. Chain termination and chain transfer ...
for the generation of bio-materials. New types of polymers are able to be constructed with unique properties, such as temperature and pH sensitivity.
Specific materials and their applications include polymer-protein and polymer-drug conjugates, mediation of enzyme activity, molecular recognition processes and polymeric micelles
A micelle () or micella () (plural micelles or micellae, respectively) is an aggregate (or supramolecular assembly) of surfactant amphipathic lipid molecules dispersed in a liquid, forming a colloidal suspension (also known as associated colloi ...
which can deliver a drug to a specific site in the body.
RAFT has also been used to graft polymer chains onto polymeric surfaces, for example, polymeric microspheres.
RAFT compared to other controlled polymerizations
Advantages
Polymerization can be performed in large range of solvents (including water), within a wide temperature range, high functional group tolerance and absent of metals for polymerization. As of 2014, the range of commercially available RAFT agents covers close to all the monomer classes that can undergo radical polymerization.Disadvantages
A particular RAFT agent is only suitable for a limited set of monomers and the synthesis of a RAFT agent typically requires a multistep synthetic procedure and subsequent purification. RAFT agents can be unstable over long time periods, are highly colored and can have a pungent odor due to gradual decomposition of the dithioester moiety to yield small sulfur compounds. The presence of sulfur and color in the resulting polymer may also be undesirable for some applications; however, this can, to an extent, be eliminated with further chemical and physical purification steps.See also
*Radical (chemistry)
In chemistry, a radical, also known as a free radical, is an atom, molecule, or ion that has at least one unpaired electron, unpaired valence electron.
With some exceptions, these unpaired electrons make radicals highly chemical reaction, chemi ...
* Copolymer
In polymer chemistry, a copolymer is a polymer derived from more than one species of monomer. The polymerization of monomers into copolymers is called copolymerization. Copolymers obtained from the copolymerization of two monomer species are some ...
* Living polymerization
In polymer chemistry, living polymerization is a form of chain growth polymerization where the ability of a growing polymer chain to terminate has been removed. This can be accomplished in a variety of ways. Chain termination and chain transfer ...
* ATRP (chemistry) Atom transfer radical polymerization (ATRP) is an example of a reversible-deactivation radical polymerization. Like its counterpart, ATRA, or atom transfer radical addition, ATRP is a means of forming a carbon-carbon bond with a transition metal cat ...
* NMP
References
{{DEFAULTSORT:Reversible Addition-Fragmentation Chain Transfer Polymerization Polymerization reactions