Resonance Ionization Mass Spectrometry on:
[Wikipedia]
[Google]
[Amazon]
 Resonance ionization is a process in
Resonance ionization is a process in
 Cesium atoms was subsequently used to show that single atoms of an element could be counted if its resonance ionization was performed in a counter in which an electron could be detected for an atom in its ground state. Subsequently, advanced techniques categorized under resonance ionization mass spectrometry (RIMS) were used to generate the relative abundance of various ion types by coupling the RIS lasers to
Cesium atoms was subsequently used to show that single atoms of an element could be counted if its resonance ionization was performed in a counter in which an electron could be detected for an atom in its ground state. Subsequently, advanced techniques categorized under resonance ionization mass spectrometry (RIMS) were used to generate the relative abundance of various ion types by coupling the RIS lasers to
Theory of Resonance Ionization Spectroscopy
In: Martellucci S., Chester A.N. (eds) ''Analytical Laser Spectroscopy.'' NATO ASI Series (Series B: Physics), vol 119. Springer, Boston, MA. * Parks J.E., Young J.P. (2000) ''Resonance Ionization Spectroscopy 2000: Laser Ionization and Applications Incorporating RIS''; 10th International Symposium, Knoxville, Tennessee (AIP Conference Proceedings). Mass spectrometry Ionization
 Resonance ionization is a process in
Resonance ionization is a process in optical physics
Atomic, molecular, and optical physics (AMO) is the study of matter-matter and light-matter interactions; at the scale of one or a few atoms and energy scales around several electron volts. The three areas are closely interrelated. AMO theor ...
used to excite a specific atom (or molecule) beyond its ionization potential to form an ion using a beam of photons irradiated from a pulsed laser light. In resonance ionization, the absorption or emission properties of the emitted photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are Massless particle, massless ...
s are not considered, rather only the resulting excited ions are mass-selected, detected and measured. Depending on the laser light source used, one electron can be removed from each atom so that resonance ionization produces an efficient selectivity in two ways: elemental selectivity in ionization and isotopic selectivity in measurement.
During resonance ionization, an ion gun creates a cloud of atoms and molecules from a gas-phase sample surface and a tunable laser
A tunable laser is a laser whose wavelength of operation can be altered in a controlled manner. While all laser gain media allow small shifts in output wavelength, only a few types of lasers allow continuous tuning over a significant wavelength ra ...
is used to fire a beam of photons at the cloud of particles emanating from the sample (analyte
An analyte, component (in clinical chemistry), or chemical species is a substance or chemical constituent that is of interest in an analytical procedure. The purest substances are referred to as analytes, such as 24 karat gold, NaCl, water, et ...
).
An initial photon from this beam is absorbed by one of the sample atoms, exciting one of the atom's electrons
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary electric charge. Electrons belong to the first generation of the lepton particle family,
and are generally thought to be elementary partic ...
to an intermediate excited state
In quantum mechanics, an excited state of a system (such as an atom, molecule or nucleus) is any quantum state of the system that has a higher energy than the ground state (that is, more energy than the absolute minimum). Excitation refers t ...
. A second photon then ionizes the same atom from the intermediate state such that its high energy level causes it to be ejected from its orbital
Orbital may refer to:
Sciences Chemistry and physics
* Atomic orbital
* Molecular orbital
* Hybrid orbital Astronomy and space flight
* Orbit
** Earth orbit
Medicine and physiology
* Orbit (anatomy), also known as the ''orbital bone''
* Orbito ...
; the result is a packet of positively charged ions which are then delivered to a mass analyzer.
Resonance ionization contrasts with resonance-enhanced multiphoton ionization (REMPI) in that the latter is neither selective nor efficient since resonances are seldom used to prevent interference. Also, resonance ionization is used for an atomic (elemental) analyte
An analyte, component (in clinical chemistry), or chemical species is a substance or chemical constituent that is of interest in an analytical procedure. The purest substances are referred to as analytes, such as 24 karat gold, NaCl, water, et ...
, whereas REMPI is used for a molecular analyte
An analyte, component (in clinical chemistry), or chemical species is a substance or chemical constituent that is of interest in an analytical procedure. The purest substances are referred to as analytes, such as 24 karat gold, NaCl, water, et ...
.
The analytical technique on which the process of resonance ionization is based is termed resonance ionization mass spectrometry (RIMS). RIMS is derived from the original method, resonance ionization spectroscopy (RIS), which was initially being used to detect single atoms with better time resolution. RIMS has proved useful in the investigation of radioactive isotopes (such as for studying rare fleeting isotopes produced in high-energy collisions), trace analysis (such as for discovering impurities in highly pure materials), atomic spectroscopy (such as for detecting low-content materials in biological samples), and for applications in which high levels of sensitivity and elemental selectivity are desired.
History
Resonance ionization was first used in aspectroscopy
Spectroscopy is the field of study that measures and interprets the electromagnetic spectra that result from the interaction between electromagnetic radiation and matter as a function of the wavelength or frequency of the radiation. Matter ...
experiment in 1971 at the Institute for Spectroscopy Russian Academy of Sciences; in that experiment, ground state rubidium
Rubidium is the chemical element with the symbol Rb and atomic number 37. It is a very soft, whitish-grey solid in the alkali metal group, similar to potassium and caesium. Rubidium is the first alkali metal in the group to have a density hig ...
atoms were ionized using ruby lasers. /sup> In 1974, a group of photophysical researchers at the Oak Ridge National Laboratory
Oak Ridge National Laboratory (ORNL) is a U.S. multiprogram science and technology national laboratory sponsored by the U.S. Department of Energy (DOE) and administered, managed, and operated by UT–Battelle as a federally funded research an ...
led by George Samuel Hurst
George Samuel Hurst (13 October 1927 – 4 July 2010) was a health physicist and professor of physics at the University of Kentucky.
Early life
Hurst was born on 13 October 1927 in the rural town of Ponza, Bell County, Kentucky located near Pin ...
developed, for the first time, the resonance ionization process on helium atoms. They wanted to use laser light to measure the number of singlet metastable helium, He (21S), particles created from energetic protons. The group achieved the selective ionization of the excited state of an atom at nearly 100% efficiency by using pulsed laser light to pass a beam of protons into the helium gas cell. The experiment on singlet metastable helium atoms was seminal in the journey towards using resonance ionization spectroscopy (RIS) for extensive atomic analysis in research settings.
 Cesium atoms was subsequently used to show that single atoms of an element could be counted if its resonance ionization was performed in a counter in which an electron could be detected for an atom in its ground state. Subsequently, advanced techniques categorized under resonance ionization mass spectrometry (RIMS) were used to generate the relative abundance of various ion types by coupling the RIS lasers to
Cesium atoms was subsequently used to show that single atoms of an element could be counted if its resonance ionization was performed in a counter in which an electron could be detected for an atom in its ground state. Subsequently, advanced techniques categorized under resonance ionization mass spectrometry (RIMS) were used to generate the relative abundance of various ion types by coupling the RIS lasers to magnetic sector
A sector instrument is a general term for a class of mass spectrometer that uses a static electric (E) or magnetic (B) sector or some combination of the two (separately in space) as a mass analyzer. Popular combinations of these sectors have been ...
, quadrupole
A quadrupole or quadrapole is one of a sequence of configurations of things like electric charge or current, or gravitational mass that can exist in ideal form, but it is usually just part of a multipole expansion of a more complex structure ref ...
, or time-of-flight (TOF) mass spectrometers.
The field of resonance ionization spectroscopy (RIS) has largely been shaped by the formal and informal communications heralding its discovery. Research papers on RIS have heavily relied on self-citation from inception, a trend which climaxed three years later with the founding of a company to commercialize the technique.
Method
A model resonance ionization mass spectrometry (RIMS) set-up consists of a laser system (consisting of multiple lasers), sample from which the atoms are derived, and a suitable mass spectrometer which mass-selectively detects the photo ions created fromresonance
Resonance describes the phenomenon of increased amplitude that occurs when the frequency of an applied periodic force (or a Fourier component of it) is equal or close to a natural frequency of the system on which it acts. When an oscillat ...
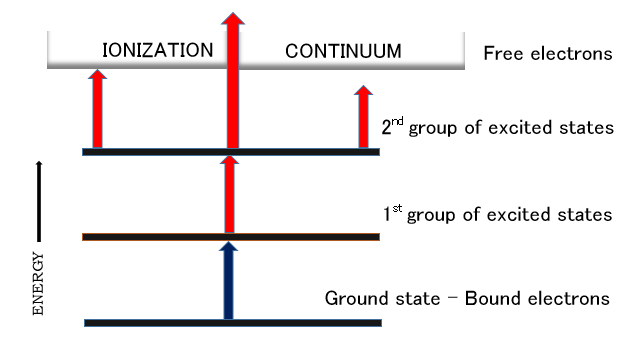
. In resonant ionization, atoms or molecules from ground state are excited to higher energy states by the resonant absorption of photons to produce ions. These ions are then monitored by appropriate detectors. In order to ensure a highly-efficient sensitivity and process saturation, the atomic or molecular beam must be formed from the ground state, the atoms should be efficiently excited and ionized, and each atom should be converted by the photon field of a short-timed pulsed laser to produce a positive ion and a valence electron.
In a basic RIS process, a pulsed laser beam produces photons of the right energy in order to excite an atom initially in its ground state, ''a'', to an excited level, ''b''. During the laser pulse, the ion population of state ''b'' increases at the expense of that of state ''a''. After a few minutes, the rate of stimulated emission from the excited state will equal rate of production so that the system is in equilibrium as long as the laser intensity is kept sufficiently high during a pulse. This high laser intensity translates into a photon fluence (photons per unit of beam area) large enough so that a necessary condition for the saturation of the RIS process has been met. If, in addition, the rate of photoionization
Photoionization is the physical process in which an ion is formed from the interaction of a photon with an atom or molecule.
Cross section
Not every interaction between a photon and an atom, or molecule, will result in photoionization. The prob ...
is greater than the rate of consumption of intermediates, then each selected state is converted to one electron plus one positive ion, so that the RIS process is saturated.
A usually efficient way to produce free atoms of an element in the ground state is to atomize the elements by ion sputtering or thermal vaporization of the element from a laser matrix under vacuum conditions or at environments with pressures significantly less than normal atmospheric pressure. The resulting plume of secondary atoms is then channeled through the path of multiple tuned laser beams which are capable of exciting consecutive electronic transitions in the specified element. Light from these tuned lasers promotes the desired atoms above their ionization potentials whereas interfering atoms from other elements are hardly ionized since they are generally transparent to the laser beam. This process produces photoions which are extracted and directed towards an analytical facility such as a magnetic sector to be counted. This approach is extremely sensitive to atoms of the specified element so that the ionization efficiency is almost 100% and also elementally selective, due to the highly unlikely chance that other species will be resonantly ionized.
To achieve high ionization efficiencies, monochromatic lasers with high instantaneous spectral power are used. Typical lasers being used include continuous-wave lasers with extremely high spectral purity and pulsed lasers for analyses involving limited atoms. Continuous-wave lasers however are often preferred to pulsed lasers due to the latter's relatively low duty cycle since they can only produce photo ions during the brief later pulses, and the difficulty in reproducing results due to pulse-to-pulse jitters, laser beam drifting, and wavelength variations.
Moderate laser powers, if high enough to affect the desired transition states, can be used since the non-resonant photoionization cross section is low which implies a negligible ionization efficiency of unwanted atoms. The influence of the laser matrix to be used for the sample can also be reduced by separating evaporation and ionization processes both in time and in space.
Another factor that could affect the efficiency and selectivity of the ionization process is the presence of contaminants caused by surface or impact ionization. This can be reduced up to appreciable orders of magnitude by using mass analysis so that isotopic compositions of the desired element are determined. Most of the elements of the Periodic Table
The periodic table, also known as the periodic table of the (chemical) elements, is a rows and columns arrangement of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of ...
can be ionized by one of the several excitation schemes available.
The suitable excitation scheme depends on certain factors including the level scheme of the element's atom, its ionization energy
Ionization, or Ionisation is the process by which an atom or a molecule acquires a negative or positive Electric charge, charge by gaining or losing electrons, often in conjunction with other chemical changes. The resulting electrically charged a ...
, required selectivity and sensitivity, likely interference, and the wavelengths and power levels of the available laser systems. Most excitation schemes vary in the last step, the ionization step. This is due to the low cross-section for non-resonant photo-ionization produced by the laser. A pulsed laser system facilitates the efficient coupling of a time-of-flight mass spectrometer (TOF-MS) to the resonance ionization set-up due to the instrument's abundance sensitivity. This is because TOF systems can produce an abundance sensitivity of up to 104 whereas magnetic mass spectrometers can only achieve up to 102.
The total selectivity in a RIS process is a combination of the sensitivities in the various resonance transitions for multiple step-wise excitations. The probability of an atom to come in contact with the resonance of another atom is about 10−5. The addition of a mass spectrometer increases this figure by a factor of 106 such that the total elemental selectivity surpasses or at least compares to that of tandem mass spectrometry
Tandem mass spectrometry, also known as MS/MS or MS2, is a technique in instrumental analysis where two or more mass analyzers are coupled together using an additional reaction step to increase their abilities to analyse chemical samples. A comm ...
(MS/MS), the most selective technique available.
Optical excitation and ionization schemes
Optical ionization schemes are developed to produce element-selective ion source for various elements. Most of the elements of the periodic table have been resonantly ionized by using one of five major optical routes based on the principle of RIMS. The routes were formed by the absorption of two or three photons to achieve excitation and ionization and are provided on the basis of optically possible transitions between atomic levels in a process called thebound-bound transition
Every atom is composed of a nucleus and one or more electrons bound to the nucleus. The nucleus is made of one or more protons and a number of neutrons. Only the most common variety of hydrogen has no neutrons.
Every solid, liquid, gas, and ...
. For an atom of the element to be promoted to a bound-continuum, the energies emitted from the photons must be within the energy range of the selected tunable lasers. Also, the ionization energy of the last emitted photon must exceed that of the atom.
The optical ionization schemes are denoted by the amount of photons necessary to make the ion pair. For the first two Schemes 1 and 2, two photons (and processes) are involved. One photon excites the atom from the ground state to an intermediate state while the second photon ionizes the atom. In Schemes 3 and 4, three photons (and processes) are involved. The first two distinct photons create consecutive bound-bound transitions within the selected atom while the third photon is absorbed for ionization. Scheme 5 is a three-photon two-intermediate-level photoionization process. After the first two photons have been absorbed by the optical energy, the third photon achieves ionization.
The RIS process can be used to ionize all elements on the periodic table, except helium and neon, using available lasers. In fact, it is possible to ionize most elements with a single laser set-up, thus enabling rapid switching from one element to another. In the early days, optical schemes from RIMS have been used to study over 70 elements and over 39 elements can be ionized with a single laser combination using a rapid computer-modulated framework that switches elements within seconds.
Applications
As an analytical technique, RIS is useful based on some of its working operations – they include extremely low detection limit so that mass of samples could be identified up to the order of 10−15, the extremely high sensitivity and elemental selectivity useful in micro- and trace analysis when coupled with mass spectrometers, and ability of the pulsed laser ion source to produce pure isobaric ion beams. A major advantage of using resonance ionization is that it is a highly selective ionization mode; it is able to target a single type of atom among a background of many types of atoms, even when said background atoms are much more abundant than the target atoms. In addition, resonance ionization incorporates the high selectivity that is desired in spectroscopy methods with ultrasensitivity, thus making resonance ionization useful when analyzing complex samples with several atomic components. Resonance ionization spectroscopy (RIS) thus has a wide range of research and industrial applications. These include characterizing the diffusion and chemical reaction of free atoms in a gas medium, solid state surface analysis using direct sampling, studying the degree of concentration variations in a dilute vapor, detecting the allowable limits of number of particles needed in a semiconductor device, and estimating the flux ofsolar neutrino
A solar neutrino is a neutrino originating from nuclear fusion in the Sun's core, and is the most common type of neutrino passing through any source observed on Earth at any particular moment. Neutrinos are elementary particles with extremely sm ...
s on Earth.
Other uses include determining high-precision values for plutonium
Plutonium is a radioactive chemical element with the symbol Pu and atomic number 94. It is an actinide metal of silvery-gray appearance that tarnishes when exposed to air, and forms a dull coating when oxidized. The element normally exhib ...
and uranium
Uranium is a chemical element with the symbol U and atomic number 92. It is a silvery-grey metal in the actinide series of the periodic table. A uranium atom has 92 protons and 92 electrons, of which 6 are valence electrons. Uranium is weakly ...
isotopes in a rapid fashion, investigating the atomic properties of technetium
Technetium is a chemical element with the symbol Tc and atomic number 43. It is the lightest element whose isotopes are all radioactive. All available technetium is produced as a synthetic element. Naturally occurring technetium is a spontaneous ...
at the ultra trace level, and capturing the concurrent excitation of stable daughter atoms with the decay of their parent atoms as is the case for alpha particle
Alpha particles, also called alpha rays or alpha radiation, consist of two protons and two neutrons bound together into a particle identical to a helium-4 nucleus. They are generally produced in the process of alpha decay, but may also be pro ...
s, beta rays
A beta particle, also called beta ray or beta radiation (symbol β), is a high-energy, high-speed electron or positron emitted by the radioactive decay of an atomic nucleus during the process of beta decay. There are two forms of beta decay, β� ...
, and positron
The positron or antielectron is the antiparticle or the antimatter counterpart of the electron. It has an electric charge of +1 '' e'', a spin of 1/2 (the same as the electron), and the same mass as an electron. When a positron collide ...
s.
RIS is now in very common use in research facilities where the quick and quantitative determination of the elemental composition of materials is important.
Pulsed laser light sources provide higher photon fluxes than continuous-wave lasers do, however the use of pulsed lasers currently limit vast applications of RIMS in two ways. One, photo ions are created only during short laser pulses, thus significantly reducing the duty cycle
A duty cycle or power cycle is the fraction of one period in which a signal or system is active. Duty cycle is commonly expressed as a percentage or a ratio. A period is the time it takes for a signal to complete an on-and-off cycle. As a formu ...
of pulsed resonance ionization mass spectrometers relative to their continuous-beam counterparts. Two, incessant drifts in laser pointing and pulse timing alongside jitters between pulses severely hamper chances of reproducibility
Reproducibility, also known as replicability and repeatability, is a major principle underpinning the scientific method. For the findings of a study to be reproducible means that results obtained by an experiment or an observational study or in a ...
.
These issues affect the extent to which resonance ionization can be used to solve some of the challenges confronted by practical analysts today; even so, applications of RIMS are replete in various traditional and emerging disciplines such as cosmochemistry
Cosmochemistry (from Greek κόσμος ''kósmos'', "universe" and χημεία ''khemeía'') or chemical cosmology is the study of the chemical composition of matter in the universe and the processes that led to those compositions. This is don ...
, medical research
Medical research (or biomedical research), also known as experimental medicine, encompasses a wide array of research, extending from " basic research" (also called ''bench science'' or ''bench research''), – involving fundamental scienti ...
, environmental chemistry
Environmental chemistry is the scientific study of the chemical and biochemical phenomena that occur in natural places. It should not be confused with green chemistry, which seeks to reduce potential pollution at its source. It can be defined as t ...
, geophysical sciences
Earth science or geoscience includes all fields of natural science related to the planet Earth. This is a branch of science dealing with the physical, chemical, and biological complex constitutions and synergistic linkages of Earth's four spheres ...
, nuclear physics
Nuclear physics is the field of physics that studies atomic nuclei and their constituents and interactions, in addition to the study of other forms of nuclear matter.
Nuclear physics should not be confused with atomic physics, which studies the ...
, genome sequencing
Whole genome sequencing (WGS), also known as full genome sequencing, complete genome sequencing, or entire genome sequencing, is the process of determining the entirety, or nearly the entirety, of the DNA sequence of an organism's genome at a ...
, and semiconductor
A semiconductor is a material which has an electrical conductivity value falling between that of a conductor, such as copper, and an insulator, such as glass. Its resistivity falls as its temperature rises; metals behave in the opposite way. ...
s.
See also
* Resonance-enhanced multiphoton ionization *Rydberg ionization spectroscopy
Rydberg ionization spectroscopy is a spectroscopy technique in which multiple photons are absorbed by an atom causing the removal of an electron to form an ion.
Resonance ionization spectroscopy
The ionization threshold energy of atoms and small ...
* Photoionization
Photoionization is the physical process in which an ion is formed from the interaction of a photon with an atom or molecule.
Cross section
Not every interaction between a photon and an atom, or molecule, will result in photoionization. The prob ...
* Atmospheric-pressure laser ionization
* Radiometric dating
Radiometric dating, radioactive dating or radioisotope dating is a technique which is used to date materials such as rocks or carbon, in which trace radioactive impurities were selectively incorporated when they were formed. The method compares t ...
* Electron excitation
Electron excitation is the transfer of a bound electron to a more energetic, but still bound state. This can be done by photoexcitation (PE), where the electron absorbs a photon and gains all its energy or by collisional excitation (CE), where ...
* Tunable laser
A tunable laser is a laser whose wavelength of operation can be altered in a controlled manner. While all laser gain media allow small shifts in output wavelength, only a few types of lasers allow continuous tuning over a significant wavelength ra ...
s
* Cosmochemistry
Cosmochemistry (from Greek κόσμος ''kósmos'', "universe" and χημεία ''khemeía'') or chemical cosmology is the study of the chemical composition of matter in the universe and the processes that led to those compositions. This is don ...
References
Patents
* * {{US patent reference, number=4,442,354, issue-date=April 10, 1984, inventor=Hurst, G. Samuel, James E. Parks, James E. & Schmitt, Harold W, title=Method of analyzing for a component in a sampleFurther reading
* Payne M.G., Hurst G.S. (1985Theory of Resonance Ionization Spectroscopy
In: Martellucci S., Chester A.N. (eds) ''Analytical Laser Spectroscopy.'' NATO ASI Series (Series B: Physics), vol 119. Springer, Boston, MA. * Parks J.E., Young J.P. (2000) ''Resonance Ionization Spectroscopy 2000: Laser Ionization and Applications Incorporating RIS''; 10th International Symposium, Knoxville, Tennessee (AIP Conference Proceedings). Mass spectrometry Ionization