Rankine–Hugoniot Conditions on:
[Wikipedia]
[Google]
[Amazon]
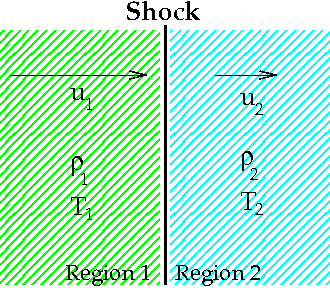 The Rankine–Hugoniot conditions, also referred to as Rankine–Hugoniot jump conditions or Rankine–Hugoniot relations, describe the relationship between the states on both sides of a
The Rankine–Hugoniot conditions, also referred to as Rankine–Hugoniot jump conditions or Rankine–Hugoniot relations, describe the relationship between the states on both sides of a
> and French engineer Pierre Henri Hugoniot.ug-87> See also: Hugoniot, H. (1889
"Mémoire sur la propagation des mouvements dans les corps et spécialement dans les gaz parfaits (deuxième partie)"
emoir on the propagation of movements in bodies, especially perfect gases (second part) ''Journal de l'École Polytechnique'', vol. 58, pages 1–125.al-06> In a coordinate system that is moving with the discontinuity, the Rankine–Hugoniot conditions can be expressed as: : where ''m'' is the mass flow rate per unit area, ''ρ''1 and ''ρ''2 are the
 The Rankine–Hugoniot conditions, also referred to as Rankine–Hugoniot jump conditions or Rankine–Hugoniot relations, describe the relationship between the states on both sides of a
The Rankine–Hugoniot conditions, also referred to as Rankine–Hugoniot jump conditions or Rankine–Hugoniot relations, describe the relationship between the states on both sides of a shock wave
In physics, a shock wave (also spelled shockwave), or shock, is a type of propagating disturbance that moves faster than the local speed of sound in the medium. Like an ordinary wave, a shock wave carries energy and can propagate through a me ...
or a combustion wave (deflagration
Deflagration (Lat: ''de + flagrare'', "to burn down") is subsonic combustion in which a pre-mixed flame propagates through a mixture of fuel and oxidizer. Deflagrations can only occur in pre-mixed fuels. Most fires found in daily life are diff ...
or detonation
Detonation () is a type of combustion involving a supersonic exothermic front accelerating through a medium that eventually drives a shock front propagating directly in front of it. Detonations propagate supersonically through shock waves with ...
) in a one-dimensional flow in fluids or a one-dimensional deformation in solids. They are named in recognition of the work carried out by Scottish engineer and physicist William John Macquorn Rankine
William John Macquorn Rankine (; 5 July 1820 – 24 December 1872) was a Scottish mechanical engineer who also contributed to civil engineering, physics and mathematics. He was a founding contributor, with Rudolf Clausius and William Thomson ( ...
an-70
The Antonov An-70 ( uk, link=no, Антонов Ан-70) is a four-engine medium-range Military transport aircraft, transport aircraft, and the first aircraft to take flight powered only by propfan engines. It was developed in the late 1980s b ..."Mémoire sur la propagation des mouvements dans les corps et spécialement dans les gaz parfaits (deuxième partie)"
emoir on the propagation of movements in bodies, especially perfect gases (second part) ''Journal de l'École Polytechnique'', vol. 58, pages 1–125.al-06> In a coordinate system that is moving with the discontinuity, the Rankine–Hugoniot conditions can be expressed as: : where ''m'' is the mass flow rate per unit area, ''ρ''1 and ''ρ''2 are the
mass density
Density (volumetric mass density or specific mass) is the substance's mass per unit of volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' can also be used. Mathematically ...
of the fluid upstream and downstream of the wave, ''u''1 and ''u''2 are the fluid velocity upstream and downstream of the wave, ''p''1 and ''p''2 are the pressures in the two regions, and ''h''1 and ''h''2 are the ''specific'' (with the sense of ''per unit mass'') enthalpies in the two regions. If in addition, the flow is reactive, then the species conservation equations demands that
:
to vanish both upstream and downstream of the discontinuity. Here, is the mass production rate of the ''i''-th species of total ''N'' species involved in the reaction. Combining conservation of mass and momentum gives us
:
which defines a straight line known as the Michelson–Rayleigh line, named after Albert A. Michelson
Albert Abraham Michelson FFRS HFRSE (surname pronunciation anglicized as "Michael-son", December 19, 1852 – May 9, 1931) was a German-born American physicist of Polish/Jewish origin, known for his work on measuring the speed of light and espe ...
and Lord Rayleigh
John William Strutt, 3rd Baron Rayleigh, (; 12 November 1842 – 30 June 1919) was an English mathematician and physicist who made extensive contributions to science. He spent all of his academic career at the University of Cambridge. Amo ...
, that has a negative slope (since is always positive) in the plane. Using the Rankine–Hugoniot equations for the conservation of mass and momentum to eliminate ''u''1 and ''u''2, the equation for the conservation of energy can be expressed as the Hugoniot equation:
:
The inverse of the density can also be expressed as the specific volume
In thermodynamics, the specific volume of a substance (symbol: , nu) is an intrinsic property of the substance, defined as the ratio of the substance's volume () to its mass (). It is the reciprocal of density (rho) and it is related to the ...
, . Along with these, one has to specify the relation between the upstream and downstream equation of state
:
where is the mass fraction of the species. Finally, the calorific equation of state is assumed to be known, i.e.,
:
Simplified Rankine–Hugoniot relations
The following assumptions are made in order to simplify the Rankine–Hugoniot equations. The mixture is assumed to obey theideal gas law
The ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas. It is a good approximation of the behavior of many gases under many conditions, although it has several limitations. It was first st ...
, so that relation between the downstream and upstream equation of state can be written as
:
where is the universal gas constant
The molar gas constant (also known as the gas constant, universal gas constant, or ideal gas constant) is denoted by the symbol or . It is the molar equivalent to the Boltzmann constant, expressed in units of energy per temperature increment per ...
and the mean molecular weight
A molecule is a group of two or more atoms held together by attractive forces known as chemical bonds; depending on context, the term may or may not include ions which satisfy this criterion. In quantum physics, organic chemistry, and bioch ...
is assumed to be constant (otherwise, would depend on the mass fraction of the all species). If one assumes that the specific heat
In thermodynamics, the specific heat capacity (symbol ) of a substance is the heat capacity of a sample of the substance divided by the mass of the sample, also sometimes referred to as massic heat capacity. Informally, it is the amount of he ...
at constant pressure is also constant across the wave, the change in enthalpies (calorific equation of state) can be simply written as
:
where the first term in the above expression represents the amount of heat released per unit mass of the upstream mixture by the wave and the second term represents the sensible heating. Eliminating temperature using the equation of state and substituting the above expression for the change in enthalpies into the Hugoniot equation, one obtains a Hugoniot equation expressed only in terms of pressure and densities,
:
where is the specific heat ratio
In thermal physics and thermodynamics, the heat capacity ratio, also known as the adiabatic index, the ratio of specific heats, or Laplace's coefficient, is the ratio of the heat capacity at constant pressure () to heat capacity at constant volu ...
. Hugoniot curve without heat release () is often called as Shock Hugoniot. Along with the Rayleigh line equation, the above equation completely determines the state of the system. These two equations can be written compactly by introducing the following non-dimensional scales,
:
The Rayleigh line equation and the Hugoniot equation then simplifies to
:
Given the upstream conditions, the intersection of above two equations in the plane determine the downstream conditions. If no heat release occurs, for example, shock waves without chemical reaction, then . The Hugoniot curves asymptote to the lines and , i.e., the pressure jump across the wave can take any values between , but the specific volume ratio is restricted to the interval (the upper bound is derived for the case because pressure cannot take negative values). The Chapman–Jouguet condition
The Chapman–Jouguet condition holds approximately in detonation waves in high explosives. It states that the detonation propagates at a velocity at which the reacting gases just reach sonic velocity (in the frame of the leading shock wave) as th ...
is where Rayleigh line is tangent to the Hugoniot curve.
If (diatomic gas without the vibrational mode excitation), the interval is , in other words, the shock wave can increase the density at most by a factor of 6. For monatomic gas, , therefore the density ratio is limited by the interval . For diatomic gases with vibrational mode excited, we have leading to the interval . In reality, the specific heat ratio is not constant in the shock wave due to molecular dissociation and ionization, but even in these cases, density ratio in general do not exceed the factor .
Derivation from Euler equations
Consider gas in a one-dimensional container (e.g., a long thin tube). Assume that the fluid isinviscid
The viscosity of a fluid is a measure of its resistance to deformation at a given rate. For liquids, it corresponds to the informal concept of "thickness": for example, syrup has a higher viscosity than water.
Viscosity quantifies the inte ...
(i.e., it shows no viscosity effects as for example friction with the tube walls). Furthermore, assume that there is no heat transfer by conduction or radiation and that gravitational acceleration can be neglected. Such a system can be described by the following system of conservation law
In physics, a conservation law states that a particular measurable property of an isolated physical system does not change as the system evolves over time. Exact conservation laws include conservation of energy, conservation of linear momentum, ...
s, known as the 1D Euler equations
200px, Leonhard Euler (1707–1783)
In mathematics and physics, many topics are named in honor of Swiss mathematician Leonhard Euler (1707–1783), who made many important discoveries and innovations. Many of these items named after Euler includ ...
, that in conservation form is:
where
* fluid mass density
Density (volumetric mass density or specific mass) is the substance's mass per unit of volume. The symbol most often used for density is ''ρ'' (the lower case Greek letter rho), although the Latin letter ''D'' can also be used. Mathematically ...
,
* fluid velocity
Velocity is the directional speed of an object in motion as an indication of its rate of change in position as observed from a particular frame of reference and as measured by a particular standard of time (e.g. northbound). Velocity i ...
,
* specific internal energy
The internal energy of a thermodynamic system is the total energy contained within it. It is the energy necessary to create or prepare the system in its given internal state, and includes the contributions of potential energy and internal kinet ...
of the fluid,
* fluid pressure
Pressure (symbol: ''p'' or ''P'') is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Gauge pressure (also spelled ''gage'' pressure)The preferred spelling varies by country a ...
, and
* is the total energy density of the fluid, /m3 while ''e'' is its specific internal energy
Assume further that the gas is calorically ideal and that therefore a polytropic equation-of-state of the simple form
is valid, where is the constant ratio of specific heats . This quantity also appears as the ''polytropic exponent'' of the polytropic process described by
For an extensive list of compressible flow equations, etc., refer to NACA
The National Advisory Committee for Aeronautics (NACA) was a United States federal agency founded on March 3, 1915, to undertake, promote, and institutionalize aeronautical research. On October 1, 1958, the agency was dissolved and its assets ...
Report 1135 (1953).me-53>
Note: For a calorically ideal gas is a constant and for a thermally ideal gas is a function of temperature. In the latter case, the dependence of pressure on mass density and internal energy might differ from that given by equation ().
The jump condition
Before proceeding further it is necessary to introduce the concept of a ''jump condition'' – a condition that holds at a discontinuity or abrupt change. Consider a 1D situation where there is a jump in the scalar conserved physical quantity , which is governed by integral conservation law for any , ,