Radical Cyclization on:
[Wikipedia]
[Google]
[Amazon]
Radical cyclization reactions are organic chemical transformations that yield cyclic products through radical intermediates. They usually proceed in three basic steps: selective radical generation, radical cyclization, and conversion of the cyclized radical to product.
 Substituents that affect the stability of these transition states can have a profound effect on the site selectivity of the reaction. Carbonyl substituents at the 2-position, for instance, encourage 6-endo ring closure. Alkyl substituents at positions 2, 3, 4, or 6 enhance selectivity for 5-exo closure.
Cyclization of the homologous 6-heptenyl radical is still selective, but is much slower—as a result, competitive side reactions are an important problem when these intermediates are involved. Additionally, 1,5-shifts can yield stabilized allylic radicals at comparable rates in these systems. In 6-hexenyl radical substrates, polarization of the reactive
Substituents that affect the stability of these transition states can have a profound effect on the site selectivity of the reaction. Carbonyl substituents at the 2-position, for instance, encourage 6-endo ring closure. Alkyl substituents at positions 2, 3, 4, or 6 enhance selectivity for 5-exo closure.
Cyclization of the homologous 6-heptenyl radical is still selective, but is much slower—as a result, competitive side reactions are an important problem when these intermediates are involved. Additionally, 1,5-shifts can yield stabilized allylic radicals at comparable rates in these systems. In 6-hexenyl radical substrates, polarization of the reactive  Cyclization reactions of vinyl, aryl, and acyl radicals are also known. Under conditions of kinetic control, 5-exo cyclization takes place preferentially. However, low concentrations of a radical scavenger establish thermodynamic control and provide access to 6-endo products—not via 6-endo cyclization, but by 5-exo cyclization followed by 3-exo closure and subsequent fragmentation (Dowd-Beckwith rearrangement). Whereas at high concentrations of the exo product is rapidly trapped preventing subsequent rearrangement to the endo product Aryl radicals exhibit similar reactivity.
''(3)''
Cyclization reactions of vinyl, aryl, and acyl radicals are also known. Under conditions of kinetic control, 5-exo cyclization takes place preferentially. However, low concentrations of a radical scavenger establish thermodynamic control and provide access to 6-endo products—not via 6-endo cyclization, but by 5-exo cyclization followed by 3-exo closure and subsequent fragmentation (Dowd-Beckwith rearrangement). Whereas at high concentrations of the exo product is rapidly trapped preventing subsequent rearrangement to the endo product Aryl radicals exhibit similar reactivity.
''(3)'' Cyclization can involve heteroatom-containing multiple bonds such as
Cyclization can involve heteroatom-containing multiple bonds such as

 Substrates with stereocenters ''between'' the radical and multiple bond are often highly stereoselective. Radical cyclizations to form polycyclic products often take advantage of this property.
Substrates with stereocenters ''between'' the radical and multiple bond are often highly stereoselective. Radical cyclizations to form polycyclic products often take advantage of this property.
 Oxidative and reductive cyclization methods also exist. These procedures require fairly electrophilic and nucleophilic radicals, respectively, to proceed effectively. Cyclic radicals are either oxidized or reduced and quenched with either external or internal nucleophiles or electrophiles, respectively.
Oxidative and reductive cyclization methods also exist. These procedures require fairly electrophilic and nucleophilic radicals, respectively, to proceed effectively. Cyclic radicals are either oxidized or reduced and quenched with either external or internal nucleophiles or electrophiles, respectively.
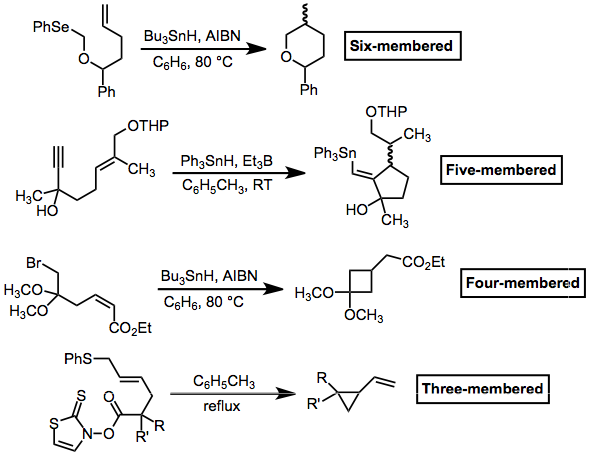 Polycycles and macrocycles can also be formed using radical cyclization reactions. In the former case, rings can be pre-formed and a single ring closed with radical cyclization, or multiple rings can be formed in a tandem process (as below). Macrocyclizations, which lack the FMO requirement of cyclizations of smaller substrates, have the unique property of exhibiting ''endo'' selectivity.
''(8)''
Polycycles and macrocycles can also be formed using radical cyclization reactions. In the former case, rings can be pre-formed and a single ring closed with radical cyclization, or multiple rings can be formed in a tandem process (as below). Macrocyclizations, which lack the FMO requirement of cyclizations of smaller substrates, have the unique property of exhibiting ''endo'' selectivity.
''(8)''
 A mixture of bromo acetal 1 (549 mg, 1.78 mmol), AIBN (30.3 mg, 0.185 mmol), and Bu3SnH (0.65 mL, 2.42 mmol) in dry
A mixture of bromo acetal 1 (549 mg, 1.78 mmol), AIBN (30.3 mg, 0.185 mmol), and Bu3SnH (0.65 mL, 2.42 mmol) in dry
Introduction
Radical cyclization reactions produce mono- or polycyclic products through the action of radical intermediates. Because they are intramolecular transformations, they are often very rapid and selective. Selective radical generation can be achieved at carbons bound to a variety offunctional group
In organic chemistry, a functional group is a substituent or moiety in a molecule that causes the molecule's characteristic chemical reactions. The same functional group will undergo the same or similar chemical reactions regardless of the res ...
s, and reagents used to effect radical generation are numerous. The radical cyclization step usually involves the attack of a radical on a multiple bond. After this step occurs, the resulting cyclized radicals are quenched through the action of a radical scavenger, a fragmentation process, or an electron-transfer reaction. Five- and six-membered rings are the most common products; formation of smaller and larger rings is rarely observed.
Three conditions must be met for an efficient radical cyclization to take place:
* A method must be available to generate a radical selectively on the substrate.
* Radical cyclization must be faster than trapping of the initially formed radical.
* All steps must be faster than undesired side reactions such as radical recombination or reaction with solvent.
Advantages: because radical intermediates are not charged species, reaction conditions are often mild and functional group tolerance is high and orthogonal to that of many polar processes. Reactions can be carried out in a variety of solvents
A solvent (s) (from the Latin '' solvō'', "loosen, untie, solve") is a substance that dissolves a solute, resulting in a solution. A solvent is usually a liquid but can also be a solid, a gas, or a supercritical fluid. Water is a solvent for ...
(including arenes, alcohols, and water), as long as the solvent does not have a weak bond that can undergo abstraction, and products are often synthetically useful compounds that can be carried on using existing functionality or groups introduced during radical trapping
Animal trapping, or simply trapping or gin, is the use of a device to remotely catch an animal. Animals may be trapped for a variety of purposes, including food, the fur trade, hunting, pest control, and wildlife management.
History
Neolithic ...
.
Disadvantages: the relative rates of the various stages of radical cyclization reactions (and any side reactions) must be carefully controlled so that cyclization and trapping of the ''cyclized'' radical is favored. Side reaction
A side reaction is a chemical reaction that occurs at the same time as the actual main reaction, but to a lesser extent. It leads to the formation of by-product, so that the yield of main product is reduced:
: + B ->[] P1
: + C ->[] P2
P1 is th ...
s are sometimes a problem, and cyclization is especially slow for small and large rings (although macrocyclizations, which resemble intermolecular radical reactions, are often high yielding).
Mechanism and stereochemistry
Prevailing mechanism
Because many reagents exist for radical generation and trapping, establishing a single prevailing mechanism is not possible. However, once a radical is generated, it can react with multiple bonds in an intramolecular fashion to yield cyclized radical intermediates. The two ends of the multiple bond constitute two possible sites of reaction. If the radical in the resulting intermediate ends up outside of the ring, the attack is termed "exo"; if it ends up inside the newly formed ring, the attack is called "endo." In many cases,exo cyclization
Baldwin's rules in organic chemistry are a series of guidelines outlining the relative favorabilities of ring closure reactions in alicyclic compounds. They were first proposed by Jack Baldwin in 1976.
Baldwin's rules discuss the relative rates ...
is favored over endo cyclization
Baldwin's rules in organic chemistry are a series of guidelines outlining the relative favorabilities of ring closure reactions in alicyclic compounds. They were first proposed by Jack Baldwin in 1976.
Baldwin's rules discuss the relative rates ...
(macrocyclizations constitute the major exception to this rule). 5-hexenyl radicals are the most synthetically useful intermediates for radical cyclizations, because cyclization is extremely rapid and exo selective. Although the exo radical is less thermodynamically stable than the endo radical, the more rapid exo cyclization is rationalized by better orbital overlap in the chair-like exo transition state (see below).
''(1)'' Substituents that affect the stability of these transition states can have a profound effect on the site selectivity of the reaction. Carbonyl substituents at the 2-position, for instance, encourage 6-endo ring closure. Alkyl substituents at positions 2, 3, 4, or 6 enhance selectivity for 5-exo closure.
Cyclization of the homologous 6-heptenyl radical is still selective, but is much slower—as a result, competitive side reactions are an important problem when these intermediates are involved. Additionally, 1,5-shifts can yield stabilized allylic radicals at comparable rates in these systems. In 6-hexenyl radical substrates, polarization of the reactive
Substituents that affect the stability of these transition states can have a profound effect on the site selectivity of the reaction. Carbonyl substituents at the 2-position, for instance, encourage 6-endo ring closure. Alkyl substituents at positions 2, 3, 4, or 6 enhance selectivity for 5-exo closure.
Cyclization of the homologous 6-heptenyl radical is still selective, but is much slower—as a result, competitive side reactions are an important problem when these intermediates are involved. Additionally, 1,5-shifts can yield stabilized allylic radicals at comparable rates in these systems. In 6-hexenyl radical substrates, polarization of the reactive double bond
In chemistry, a double bond is a covalent bond between two atoms involving four bonding electrons as opposed to two in a single bond. Double bonds occur most commonly between two carbon atoms, for example in alkenes. Many double bonds exist betw ...
with electron-withdrawing functional group
In chemistry, an electron-withdrawing group (EWG) is a substituent that has some of the following kinetic and thermodynamic implications:
*with regards to electron transfer, electron-withdrawing groups enhance the oxidizing power tendency of the ...
s is often necessary to achieve high yields. Stabilizing the ''initially formed'' radical with electron-withdrawing groups provides access to more stable 6-endo cyclization products preferentially.
''(2)'' Cyclization reactions of vinyl, aryl, and acyl radicals are also known. Under conditions of kinetic control, 5-exo cyclization takes place preferentially. However, low concentrations of a radical scavenger establish thermodynamic control and provide access to 6-endo products—not via 6-endo cyclization, but by 5-exo cyclization followed by 3-exo closure and subsequent fragmentation (Dowd-Beckwith rearrangement). Whereas at high concentrations of the exo product is rapidly trapped preventing subsequent rearrangement to the endo product Aryl radicals exhibit similar reactivity.
''(3)''
Cyclization reactions of vinyl, aryl, and acyl radicals are also known. Under conditions of kinetic control, 5-exo cyclization takes place preferentially. However, low concentrations of a radical scavenger establish thermodynamic control and provide access to 6-endo products—not via 6-endo cyclization, but by 5-exo cyclization followed by 3-exo closure and subsequent fragmentation (Dowd-Beckwith rearrangement). Whereas at high concentrations of the exo product is rapidly trapped preventing subsequent rearrangement to the endo product Aryl radicals exhibit similar reactivity.
''(3)'' Cyclization can involve heteroatom-containing multiple bonds such as
Cyclization can involve heteroatom-containing multiple bonds such as nitrile
In organic chemistry, a nitrile is any organic compound that has a functional group. The prefix '' cyano-'' is used interchangeably with the term ''nitrile'' in industrial literature. Nitriles are found in many useful compounds, including me ...
s, oxime
In organic chemistry, an oxime is a organic compound belonging to the imines, with the general formula , where R is an organic side-chain and R’ may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substitu ...
s, and carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containin ...
s. Attack at the carbon atom of the multiple bond is almost always observed. In the latter case attack is reversible; however alkoxy radicals can be trapped using a stannane trapping agent.
Stereoselectivity
The diastereoselectivity of radical cyclizations is often high. In most all-carbon cases, selectivity can be rationalized according to Beckwith's guidelines, which invoke the reactant-like, exotransition state
In chemistry, the transition state of a chemical reaction is a particular configuration along the reaction coordinate. It is defined as the state corresponding to the highest potential energy along this reaction coordinate. It is often marked ...
shown above. Placing substituents in pseudoequatorial positions in the transition state leads to ''cis'' products from simple secondary radicals. Introducing polar substituents can favor ''trans'' products due to steric or electronic repulsion between the polar groups. In more complex systems, the development of transition state models requires consideration of factors such as allylic strain and boat-like transition states
''(4)''
Chiral auxiliaries
In stereochemistry, a chiral auxiliary is a Stereogenic center, stereogenic group or unit that is temporarily incorporated into an organic compound in order to control the stereochemical outcome of the synthesis. The chirality present in the auxil ...
have been used in enantioselective radical cyclizations with limited success. Small energy differences between early transition states constitute a profound barrier to success in this arena. In the example shown, diastereoselectivity (for both configurations of the left-hand stereocenter) is low and enantioselectivity is only moderate.
''(5)'' Substrates with stereocenters ''between'' the radical and multiple bond are often highly stereoselective. Radical cyclizations to form polycyclic products often take advantage of this property.
Substrates with stereocenters ''between'' the radical and multiple bond are often highly stereoselective. Radical cyclizations to form polycyclic products often take advantage of this property.
Scope and limitations
Radical generation methods
The use ofmetal hydride
In chemistry, a hydride is formally the anion of hydrogen( H−). The term is applied loosely. At one extreme, all compounds containing covalently bound H atoms are called hydrides: water (H2O) is a hydride of oxygen, ammonia is a hydride of ...
s ( tin, silicon
Silicon is a chemical element with the symbol Si and atomic number 14. It is a hard, brittle crystalline solid with a blue-grey metallic luster, and is a tetravalent metalloid and semiconductor. It is a member of group 14 in the periodic ...
and mercury hydrides) is common in radical cyclization reactions; the primary limitation of this method is the possibility of reduction of the initially formed radical by H-M. Fragmentation methods avoid this problem by incorporating the chain-transfer reagent
Chain transfer is a polymerization reaction by which the activity of a growing polymer chain is transferred to another molecule.
:P• + XR' → PX + R'•
Chain transfer reactions reduce the average molecular weight of the final polymer. ...
into the substrate itself—the active chain-carrying radical is not released until after cyclization has taken place. The products of fragmentation methods retain a double bond as a result, and extra synthetic steps are usually required to incorporate the chain-carrying group.
Atom-transfer methods rely on the movement of an atom from the acyclic starting material to the cyclic radical to generate the product. These methods use catalytic amounts of weak reagents, preventing problems associated with the presence of strong reducing agents (such as tin hydride). Hydrogen- and halogen-transfer processes are known; the latter tend to be more synthetically useful.
''(6)'' Oxidative and reductive cyclization methods also exist. These procedures require fairly electrophilic and nucleophilic radicals, respectively, to proceed effectively. Cyclic radicals are either oxidized or reduced and quenched with either external or internal nucleophiles or electrophiles, respectively.
Oxidative and reductive cyclization methods also exist. These procedures require fairly electrophilic and nucleophilic radicals, respectively, to proceed effectively. Cyclic radicals are either oxidized or reduced and quenched with either external or internal nucleophiles or electrophiles, respectively.
Ring sizes
In general, radical cyclization to produce small rings is difficult. However, it is possible to trap the cyclized radical before re-opening. This process can be facilitated by fragmentation (see the three-membered case below) or by stabilization of the cyclized radical (see the four-membered case). Five- and six-membered rings are the most common sizes produced by radical cyclization. ''(7)'' Polycycles and macrocycles can also be formed using radical cyclization reactions. In the former case, rings can be pre-formed and a single ring closed with radical cyclization, or multiple rings can be formed in a tandem process (as below). Macrocyclizations, which lack the FMO requirement of cyclizations of smaller substrates, have the unique property of exhibiting ''endo'' selectivity.
''(8)''
Polycycles and macrocycles can also be formed using radical cyclization reactions. In the former case, rings can be pre-formed and a single ring closed with radical cyclization, or multiple rings can be formed in a tandem process (as below). Macrocyclizations, which lack the FMO requirement of cyclizations of smaller substrates, have the unique property of exhibiting ''endo'' selectivity.
''(8)''
Comparison with other methods
In comparison to cationic cyclizations, radical cyclizations avoid issues associated with Wagner-Meerwein rearrangements, do not require strongly acidic conditions, and can be kinetically controlled. Cationic cyclizations are usually thermodynamically controlled. Radical cyclizations are much faster than analogous anionic cyclizations, and avoid β-elimination side reactions. Anionic Michael-type cyclization is an alternative to radical cyclization of activated olefins. Metal-catalyzed cyclization reactions usually require mildly basic conditions, and substrates must be chosen to avoid β-hydride elimination. The primary limitation of radical cyclizations with respect to these other methods is the potential for radical side reactions.Experimental conditions and procedure
Typical conditions
Radical reactions must be carried out under inert atmosphere as dioxygen is a triplet radical which will intercept radical intermediates. Because the relative rates of a number of processes are important to the reaction, concentrations must be carefully adjusted to optimize reaction conditions. Reactions are generally carried out in solvents whose bonds have high bond dissociation energies (BDEs), including benzene, methanol or benzotrifluoride. Even aqueous conditions are tolerated, since water has a strong O-H bond with a BDE of 494 kJ/mol. This is in contrast to many polar processes, where hydroxylic solvents (or polar X-H bonds in the substrate itself) may not be tolerated due to the nucleophilicity or acidity of the functional group.Example procedure
''(9)'' A mixture of bromo acetal 1 (549 mg, 1.78 mmol), AIBN (30.3 mg, 0.185 mmol), and Bu3SnH (0.65 mL, 2.42 mmol) in dry
A mixture of bromo acetal 1 (549 mg, 1.78 mmol), AIBN (30.3 mg, 0.185 mmol), and Bu3SnH (0.65 mL, 2.42 mmol) in dry benzene
Benzene is an organic chemical compound with the molecular formula C6H6. The benzene molecule is composed of six carbon atoms joined in a planar ring with one hydrogen atom attached to each. Because it contains only carbon and hydrogen ato ...
(12 mL) was heated under reflux for 1 hour and then evaporated under reduced pressure. Silicagel column chromatography of the crude product with hexane
Hexane () is an organic compound, a straight-chain alkane with six carbon atoms and has the molecular formula C6H14.
It is a colorless liquid, odorless when pure, and with boiling points approximately . It is widely used as a cheap, relatively ...
– EtOAc (92:8) as eluant gave tetrahydropyran 2 (395 mg, 97%) as an oily mixture of two diastereomers. (c 0.43, CHCl3); IR ( CHCl3):1732 cm–1;1H NMR (CDCl3)δ 4.77–4.89 (m, 0.6H), 4.66–4.69 (m, 0.4H), 3.40–4.44 (m, 4H), 3.68 (s, 3H), 2.61 (dd, J = 15.2, 4.2 Hz, 1H), 2.51 (dd, J = 15.2, 3.8 Hz, 1H), 0.73–1.06 (m, 3H); mass spectrum
A mass spectrum is a histogram plot of intensity vs. '' mass-to-charge ratio'' (''m/z'') in a chemical sample, usually acquired using an instrument called a ''mass spectrometer''. Not all mass spectra of a given substance are the same; for examp ...
: m/z 215 (M+–Me); Anal. Calcd for C12H22O4: C, 62.6; H, 9.65. Found: C, 62.6; H, 9.7.Ikara, M.; Yasai, K.; Tanigachi, N.; Fukumoto, K. ''J. Chem. Soc., Perkin Trans. 1'', 1990, 1469.
References
{{reflist, 30em Organic reactions