Quelet Reaction on:
[Wikipedia]
[Google]
[Amazon]
The Quelet reaction (also called the Blanc–Quelet reaction) is an organic coupling reaction in which a phenolic ether reacts with an aliphatic aldehyde to generate an α-chloroalkyl derivative. The Quelet reaction is an example of a larger class of reaction,  The reaction proceeds under strong acid catalysis using HCl; zinc(II) chloride may be used as a catalyst in instances where the ether is deactivated. The reaction primarily yields para-substituted products; however it can also produce ortho-substituted compounds if the para site is blocked.
The reaction proceeds under strong acid catalysis using HCl; zinc(II) chloride may be used as a catalyst in instances where the ether is deactivated. The reaction primarily yields para-substituted products; however it can also produce ortho-substituted compounds if the para site is blocked.
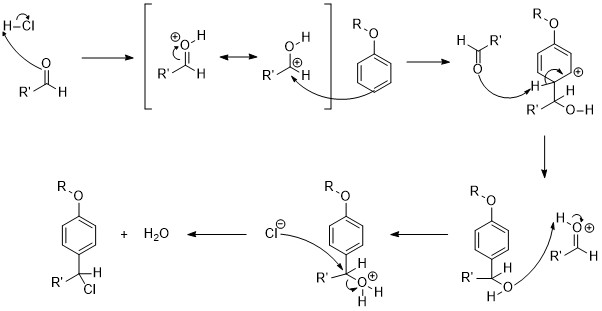 The mechanism of the Quelet reaction is primarily categorized as a reaction in polar acid. First, the
The mechanism of the Quelet reaction is primarily categorized as a reaction in polar acid. First, the
 The reaction requires a
The reaction requires a
electrophilic aromatic substitution
Electrophilic aromatic substitution is an organic reaction in which an atom that is attached to an aromatic system (usually hydrogen) is replaced by an electrophile. Some of the most important electrophilic aromatic substitutions are aromatic ni ...
. The reaction is named after its creator R. Quelet, who first reported the reaction in 1932, and is similar to the Blanc chloromethylation
The Blanc chloromethylation (also called the Blanc reaction) is the chemical reaction of aromatic rings with formaldehyde and hydrogen chloride to form chloromethyl arenes. The reaction is catalyzed by Lewis acids such as zinc chloride. The ...
process.
 The reaction proceeds under strong acid catalysis using HCl; zinc(II) chloride may be used as a catalyst in instances where the ether is deactivated. The reaction primarily yields para-substituted products; however it can also produce ortho-substituted compounds if the para site is blocked.
The reaction proceeds under strong acid catalysis using HCl; zinc(II) chloride may be used as a catalyst in instances where the ether is deactivated. The reaction primarily yields para-substituted products; however it can also produce ortho-substituted compounds if the para site is blocked.
Mechanism
 The mechanism of the Quelet reaction is primarily categorized as a reaction in polar acid. First, the
The mechanism of the Quelet reaction is primarily categorized as a reaction in polar acid. First, the carbonyl
In organic chemistry, a carbonyl group is a functional group composed of a carbon atom double-bonded to an oxygen atom: C=O. It is common to several classes of organic compounds, as part of many larger functional groups. A compound containin ...
is protonated
In chemistry, protonation (or hydronation) is the adding of a proton (or hydron, or hydrogen cation), (H+) to an atom, molecule, or ion, forming a conjugate acid. (The complementary process, when a proton is removed from a Brønsted–Lowry acid ...
forming a highly reactive protonated aldehyde
In organic chemistry, an aldehyde () is an organic compound containing a functional group with the structure . The functional group itself (without the "R" side chain) can be referred to as an aldehyde but can also be classified as a formyl group ...
that acts as the electrophile
In chemistry, an electrophile is a chemical species that forms bonds with nucleophiles by accepting an electron pair. Because electrophiles accept electrons, they are Lewis acids. Most electrophiles are positively charged, have an atom that ca ...
to the nucleophilic
In chemistry, a nucleophile is a chemical species that forms bonds by donating an electron pair. All molecules and ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Because nucleophiles donate electrons, they a ...
pi-bond of the aromatic
In chemistry, aromaticity is a chemical property of cyclic (ring-shaped), ''typically'' planar (flat) molecular structures with pi bonds in resonance (those containing delocalized electrons) that gives increased stability compared to sat ...
ring. Next, the aromatic ring is reformed via E1. Finally, the hydroxy group
In chemistry, a hydroxy or hydroxyl group is a functional group with the chemical formula and composed of one oxygen atom covalently bonded to one hydrogen atom. In organic chemistry, alcohols and carboxylic acids contain one or more hydrox ...
formed from the carbonyl oxygen is protonated a second time and leaves as a molecule of water, creating a carbocation
A carbocation is an ion with a positively charged carbon atom. Among the simplest examples are the methenium , methanium and vinyl cations. Occasionally, carbocations that bear more than one positively charged carbon atom are also encoun ...
that is attacked by the negatively charged chlorine ion.
Reaction conditions and limitations
 The reaction requires a
The reaction requires a strong acid
Acid strength is the tendency of an acid, symbolised by the chemical formula HA, to dissociate into a proton, H+, and an anion, A-. The dissociation of a strong acid in solution is effectively complete, except in its most concentrated solutio ...
catalyst, but both Lewis acids and Brownsted-Lowry acids can be used in the Quelet reaction. It has been noted that aqueous formaldehyde sometimes produces a better yield than paraformaldehyde
Paraformaldehyde (PFA) is the smallest polyoxymethylene, the polymerization product of formaldehyde with a typical degree of polymerization of 8–100 units. Paraformaldehyde commonly has a slight odor of formaldehyde due to decomposition. Paraf ...
. The reaction was first reported using zinc(II) chloride, however the reaction has been noted to proceed in the absence of this catalyst in highly activated aromatic compounds. If using an aromatic compound where the para-site is blocked, the reaction will add in the ortho-position (see example right).
Not all aromatic compounds can undergo Quelet reactions. For example, too highly halogenated aromatic compounds, aromatic compounds with nitro groups, and terphenyl
Terphenyls are a group of closely related aromatic hydrocarbons. Also known as diphenylbenzenes or triphenyls, they consist of a central benzene ring substituted with two phenyl groups. There are three substitution patterns: ''ortho''-terpheny ...
s cannot be used as reactants for Quelet reactions. Even for compounds that can undergo Quelet reactions, there sometimes exists other reactions that produce the same products in higher yields. The Quelet reaction can produce dangerous halomethyl ethers, gaseous and liquid compounds that are toxic to humans, and therefore is sometimes passed up for chloromethylations without these harmful biproducts.
Usage
The Quelet reaction is an important step in thepolymerization
In polymer chemistry, polymerization (American English), or polymerisation (British English), is a process of reacting monomer molecules together in a chemical reaction to form polymer chains or three-dimensional networks. There are many fo ...
of aromatic monomer
In chemistry, a monomer ( ; ''mono-'', "one" + '' -mer'', "part") is a molecule that can react together with other monomer molecules to form a larger polymer chain or three-dimensional network in a process called polymerization.
Classification
...
s, such as styrene
Styrene () is an organic compound with the chemical formula C6H5CH=CH2. This derivative of benzene is a colorless oily liquid, although aged samples can appear yellowish. The compound evaporates easily and has a sweet smell, although high concen ...
, PPO and PPEK. These chloromethylated aromatic polymers are used in a diverse set of industries, such as fuel cell
A fuel cell is an electrochemical cell that converts the chemical energy of a fuel (often hydrogen fuel, hydrogen) and an oxidizing agent (often oxygen) into electricity through a pair of redox reactions. Fuel cells are different from most bat ...
s and membranes for drug delivery
Drug delivery refers to approaches, formulations, manufacturing techniques, storage systems, and technologies involved in transporting a pharmaceutical compound to its target site to achieve a desired therapeutic effect. Principles related to dr ...
.
See also
* Blanc reaction *Electrophilic aromatic substitution
Electrophilic aromatic substitution is an organic reaction in which an atom that is attached to an aromatic system (usually hydrogen) is replaced by an electrophile. Some of the most important electrophilic aromatic substitutions are aromatic ni ...
* Friedel-Crafts Alkylation
References
{{Reflist Name reactions Addition reactions Substitution reactions Carbon-carbon bond forming reactions