Quantum Biology on:
[Wikipedia]
[Google]
[Amazon]
Quantum biology is the study of applications of
File:Macrophage ferritin.jpg, Electron microscope image of placental macrophage ferritin
File:Ferritin_tunneling (cropped).tif, Conductive atomic force microscopy image of human substantia nigra pars compacta (SNc) tissue
File:Iron_outside_of_neuroemlanin.png, Electron spectroscopic imaging of iron (red) outside of neuromelanin organelles
File:Glial_cell.png, Electron microscope image of glial cell from SNc showing structures similar to ferritin in placental tissue
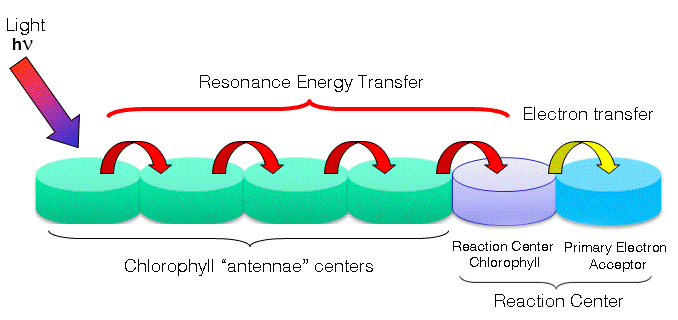

 Photosynthesis refers to the biological process that photosynthetic cells use to synthesize organic compounds from inorganic starting materials using sunlight. What has been primarily implicated as exhibiting non-trivial quantum behaviors is the light reaction stage of photosynthesis. In this stage, photons are absorbed by the membrane-bound photosystems. Photosystems contain two major domains, the light-harvesting complex (antennae) and the reaction center. These antennae vary among organisms. For example, bacteria use circular aggregates of chlorophyll pigments, while plants use membrane-embedded protein and chlorophyll complexes. Regardless, photons are first captured by the antennae and passed on to the reaction-center complex. Various pigment-protein complexes, such as the FMO complex in green sulfur bacteria, are responsible for transferring energy from antennae to reaction site. The photon-driven excitation of the reaction-center complex mediates the oxidation and the reduction of the primary electron acceptor, a component of the reaction-center complex. Much like the
Photosynthesis refers to the biological process that photosynthetic cells use to synthesize organic compounds from inorganic starting materials using sunlight. What has been primarily implicated as exhibiting non-trivial quantum behaviors is the light reaction stage of photosynthesis. In this stage, photons are absorbed by the membrane-bound photosystems. Photosystems contain two major domains, the light-harvesting complex (antennae) and the reaction center. These antennae vary among organisms. For example, bacteria use circular aggregates of chlorophyll pigments, while plants use membrane-embedded protein and chlorophyll complexes. Regardless, photons are first captured by the antennae and passed on to the reaction-center complex. Various pigment-protein complexes, such as the FMO complex in green sulfur bacteria, are responsible for transferring energy from antennae to reaction site. The photon-driven excitation of the reaction-center complex mediates the oxidation and the reduction of the primary electron acceptor, a component of the reaction-center complex. Much like the
 Magnetoreception is the ability of animals to navigate using the inclination of the magnetic field of the Earth. A possible explanation for magnetoreception is the entangled radical pair mechanism. The radical-pair mechanism is well-established in
Magnetoreception is the ability of animals to navigate using the inclination of the magnetic field of the Earth. A possible explanation for magnetoreception is the entangled radical pair mechanism. The radical-pair mechanism is well-established in
Philip Ball (2015). "Quantum Biology: An Introduction". The Royal Institution
Quantum Biology and the Hidden Nature of Nature, World Science Festival 2012, video of podium discussion
Quantum Biology: Current Status and Opportunities, September 17-18, 2012, University of Surrey, UK
{{DEFAULTSORT:Quantum Biology Biophysics
quantum mechanics
Quantum mechanics is the fundamental physical Scientific theory, theory that describes the behavior of matter and of light; its unusual characteristics typically occur at and below the scale of atoms. Reprinted, Addison-Wesley, 1989, It is ...
and theoretical chemistry
Theoretical chemistry is the branch of chemistry which develops theoretical generalizations that are part of the theoretical arsenal of modern chemistry: for example, the concepts of chemical bonding, chemical reaction, valence, the surface ...
to aspects of biology
Biology is the scientific study of life and living organisms. It is a broad natural science that encompasses a wide range of fields and unifying principles that explain the structure, function, growth, History of life, origin, evolution, and ...
that cannot be accurately described by the classical laws of physics. An understanding of fundamental quantum interactions is important because they determine the properties of the next level of organization in biological systems.
Many biological processes involve the conversion of energy
Energy () is the physical quantity, quantitative physical property, property that is transferred to a physical body, body or to a physical system, recognizable in the performance of Work (thermodynamics), work and in the form of heat and l ...
into forms that are usable for chemical transformations, and are quantum mechanical in nature. Such processes involve chemical reactions
A chemical reaction is a process that leads to the chemical transformation of one set of chemical substances to another. When chemical reactions occur, the atoms are rearranged and the reaction is accompanied by an energy change as new products ...
, light absorption, formation of excited electronic states, transfer of excitation energy, and the transfer of electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
s and proton
A proton is a stable subatomic particle, symbol , Hydron (chemistry), H+, or 1H+ with a positive electric charge of +1 ''e'' (elementary charge). Its mass is slightly less than the mass of a neutron and approximately times the mass of an e ...
s (hydrogen ion
A hydrogen ion is created when a hydrogen atom loses or gains an electron. A positively charged hydrogen ion (or proton) can readily combine with other particles and therefore is only seen isolated when it is in a gaseous state or a nearly particl ...
s) in chemical processes, such as photosynthesis
Photosynthesis ( ) is a system of biological processes by which photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical energy necessary to fuel their metabo ...
, visual perception
Visual perception is the ability to detect light and use it to form an image of the surrounding Biophysical environment, environment. Photodetection without image formation is classified as ''light sensing''. In most vertebrates, visual percept ...
, olfaction
The sense of smell, or olfaction, is the special sense through which smells (or odors) are perceived. The sense of smell has many functions, including detecting desirable foods, hazards, and pheromones, and plays a role in taste.
In humans, ...
, and cellular respiration
Cellular respiration is the process of oxidizing biological fuels using an inorganic electron acceptor, such as oxygen, to drive production of adenosine triphosphate (ATP), which stores chemical energy in a biologically accessible form. Cell ...
. Moreover, quantum biology may use computations to model biological interactions in light of quantum mechanical effects. Quantum biology is concerned with the influence of non-trivial quantum phenomena, which can be explained by reducing the biological
Biology is the scientific study of life and living organisms. It is a broad natural science that encompasses a wide range of fields and unifying principles that explain the structure, function, growth, origin, evolution, and distribution of ...
process to fundamental physics
Physics is the scientific study of matter, its Elementary particle, fundamental constituents, its motion and behavior through space and time, and the related entities of energy and force. "Physical science is that department of knowledge whi ...
, although these effects are difficult to study and can be speculative.
Currently, there exist four major life processes that have been identified as influenced by quantum effects: enzyme catalysis, sensory processes, energy transference, and information encoding.
History
Quantum biology is an emerging field, in the sense that most current research is theoretical and subject to questions that require further experimentation. Though the field has only recently received an influx of attention, it has been conceptualized by physicists throughout the 20th century. It has been suggested that quantum biology might play a critical role in the future of the medical world. Early pioneers of quantum physics saw applications of quantum mechanics in biological problems.Erwin Schrödinger
Erwin Rudolf Josef Alexander Schrödinger ( ; ; 12 August 1887 – 4 January 1961), sometimes written as or , was an Austrian-Irish theoretical physicist who developed fundamental results in quantum field theory, quantum theory. In particul ...
's 1944 book '' What Is Life?'' discussed applications of quantum mechanics in biology. Schrödinger introduced the idea of an " aperiodic crystal" that contained genetic information in its configuration of covalent chemical bond
A chemical bond is the association of atoms or ions to form molecules, crystals, and other structures. The bond may result from the electrostatic force between oppositely charged ions as in ionic bonds or through the sharing of electrons a ...
s. He further suggested that mutation
In biology, a mutation is an alteration in the nucleic acid sequence of the genome of an organism, virus, or extrachromosomal DNA. Viral genomes contain either DNA or RNA. Mutations result from errors during DNA or viral replication, ...
s are introduced by "quantum leaps". Other pioneers Niels Bohr
Niels Henrik David Bohr (, ; ; 7 October 1885 – 18 November 1962) was a Danish theoretical physicist who made foundational contributions to understanding atomic structure and old quantum theory, quantum theory, for which he received the No ...
, Pascual Jordan
Ernst Pascual Jordan (; 18 October 1902 – 31 July 1980) was a German theoretical and mathematical physicist who made significant contributions to quantum mechanics and quantum field theory. He contributed much to the mathematical form of matri ...
, and Max Delbrück argued that the quantum idea of complementarity was fundamental to the life sciences. In 1963, Per-Olov Löwdin
Per-Olov Löwdin (October 28, 1916 – October 6, 2000) was a Swedish physicist, professor at the University of Uppsala from 1960 to 1983, and in parallel at the University of Florida until 1993.
A former graduate student under Ivar Waller, Löw ...
published proton tunneling as another mechanism for DNA
Deoxyribonucleic acid (; DNA) is a polymer composed of two polynucleotide chains that coil around each other to form a double helix. The polymer carries genetic instructions for the development, functioning, growth and reproduction of al ...
mutation. In his paper, he stated that there is a new field of study called "quantum biology". In 1979, the Soviet and Ukrainian physicist Alexander Davydov published the first textbook on quantum biology entitled ''Biology and Quantum Mechanics''.
Enzyme catalysis
Enzyme
An enzyme () is a protein that acts as a biological catalyst by accelerating chemical reactions. The molecules upon which enzymes may act are called substrate (chemistry), substrates, and the enzyme converts the substrates into different mol ...
s have been postulated to use quantum tunneling
In physics, a quantum (: quanta) is the minimum amount of any physical entity (physical property) involved in an interaction. The fundamental notion that a property can be "quantized" is referred to as "the hypothesis of quantization". This me ...
to transfer electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
s in electron transport chain
An electron transport chain (ETC) is a series of protein complexes and other molecules which transfer electrons from electron donors to electron acceptors via redox reactions (both reduction and oxidation occurring simultaneously) and couples th ...
s. It is possible that protein quaternary architectures may have adapted to enable sustained quantum entanglement
Quantum entanglement is the phenomenon where the quantum state of each Subatomic particle, particle in a group cannot be described independently of the state of the others, even when the particles are separated by a large distance. The topic o ...
and coherence, which are two of the limiting factors for quantum tunneling in biological entities. These architectures might account for a greater percentage of quantum energy transfer, which occurs through electron transport and proton tunneling (usually in the form of hydrogen ions, H+). Tunneling refers to the ability of a subatomic particle to travel through potential energy barriers. This ability is due, in part, to the principle of complementarity, which holds that certain substances have pairs of properties that cannot be measured separately without changing the outcome of measurement. Particles, such as electrons and protons, have wave-particle duality; they can pass through energy barriers due to their wave characteristics without violating the laws of physics. In order to quantify how quantum tunneling is used in many enzymatic activities, many biophysicists utilize the observation of hydrogen ions. When hydrogen ions are transferred, this is seen as a staple in an organelle's primary energy processing network; in other words, quantum effects are most usually at work in proton distribution sites at distances on the order of an angstrom
The angstrom (; ) is a unit of length equal to m; that is, one ten-billionth of a metre, a hundred-millionth of a centimetre, 0.1 nanometre, or 100 picometres. The unit is named after the Swedish physicist Anders Jonas Ångström (1814–18 ...
(1 Å). In physics, a semiclassical (SC) approach is most useful in defining this process because of the transfer from quantum elements (e.g. particles) to macroscopic phenomena (e.g. biochemicals). Aside from hydrogen tunneling, studies also show that electron transfer between redox
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is t ...
centers through quantum tunneling plays an important role in enzymatic activity of photosynthesis
Photosynthesis ( ) is a system of biological processes by which photosynthetic organisms, such as most plants, algae, and cyanobacteria, convert light energy, typically from sunlight, into the chemical energy necessary to fuel their metabo ...
and cellular respiration
Cellular respiration is the process of oxidizing biological fuels using an inorganic electron acceptor, such as oxygen, to drive production of adenosine triphosphate (ATP), which stores chemical energy in a biologically accessible form. Cell ...
(see also Mitochondria section below).
Ferritin
Ferritin
Ferritin is a universal intracellular and extracellular protein that stores iron and releases it in a controlled fashion. The protein is produced by almost all living organisms, including archaea, bacteria, algae, higher plants, and animals. ...
is an iron storage protein that is found in plants and animals. It is usually formed from 24 subunits that self-assemble into a spherical shell that is approximately 2 nm thick, with an outer diameter that varies with iron loading up to about 16 nm. Up to ~4500 iron atoms can be stored inside the core of the shell in the Fe3+ oxidation state as water-insoluble compounds such as ferrihydrite
Ferrihydrite (Fh) is a widespread hydrous ferric oxyhydroxide mineral at the Earth's surface, and a likely constituent in extraterrestrial materials. It forms in several types of environments, from freshwater to marine systems, aquifers to hydro ...
and magnetite
Magnetite is a mineral and one of the main iron ores, with the chemical formula . It is one of the iron oxide, oxides of iron, and is ferrimagnetism, ferrimagnetic; it is attracted to a magnet and can be magnetization, magnetized to become a ...
. Ferritin is able to store electrons for at least several hours, which reduce the Fe3+ to water soluble Fe2+. Electron tunneling as the mechanism by which electrons transit the 2 nm thick protein shell was proposed as early as 1988. Electron tunneling and other quantum mechanical properties of ferritin were observed in 1992, and electron tunneling at room temperature and ambient conditions was observed in 2005. Electron tunneling associated with ferritin is a quantum biological process, and ferritin is a quantum biological agent.
Electron tunneling through ferritin between electrodes is independent of temperature, which indicates that it is substantially coherent and activation-less. The electron tunneling distance is a function of the size of the ferritin. Single electron tunneling events can occur over distances of up to 8 nm through the ferritin, and sequential electron tunneling can occur up to 12 nm through the ferritin. It has been proposed that the electron tunneling is magnon-assisted and associated with magnetite microdomains in the ferritin core.
Early evidence of quantum mechanical properties exhibited by ferritin ''in vivo'' was reported in 2004, where increased magnetic ordering of ferritin structures in placental macrophages was observed using small angle neutron scattering (SANS). Quantum dot solids also show increased magnetic ordering in SANS testing, and can conduct electrons over long distances. Increased magnetic ordering of ferritin cores disposed in an ordered layer on a silicon substrate with SANS testing has also been observed. Ferritin structures like those in placental macrophages have been tested in solid state configurations and exhibit quantum dot solid-like properties of conducting electrons over distances of up to 80 microns through sequential tunneling and formation of Coulomb blockades. Electron transport through ferritin in placental macrophages may be associated with an anti-inflammatory function.
Conductive atomic force microscopy of substantia nigra pars compacta (SNc) tissue demonstrated evidence of electron tunneling between ferritin cores, in structures that correlate to layers of ferritin outside of neuromelanin organelles.
Evidence of ferritin layers in cell bodies of large dopamine neurons of the SNc and between those cell bodies in glial cells has also been found, and is hypothesized to be associated with neuron function. Overexpression of ferritin reduces the accumulation of reactive oxygen species
In chemistry and biology, reactive oxygen species (ROS) are highly Reactivity (chemistry), reactive chemicals formed from diatomic oxygen (), water, and hydrogen peroxide. Some prominent ROS are hydroperoxide (H2O2), superoxide (O2−), hydroxyl ...
(ROS), and may act as a catalyst by increasing the ability of electrons from antioxidants to neutralize ROS through electron tunneling. Ferritin has also been observed in ordered configurations in lysosome
A lysosome () is a membrane-bound organelle that is found in all mammalian cells, with the exception of red blood cells (erythrocytes). There are normally hundreds of lysosomes in the cytosol, where they function as the cell’s degradation cent ...
s associated with erythropoiesis
Erythropoiesis (from Greek ''erythro'', meaning ''red'' and ''poiesis'', meaning ''to make'') is the process which produces red blood cells (erythrocytes), which is the development from erythropoietic stem cell to mature red blood cell.
It is s ...
, where it may be associated with red blood cell production. While direct evidence of tunneling associated with ferritin ''in vivo'' in live cells has not yet been obtained, it may be possible to do so using QDs tagged with anti-ferritin, which should emit photons if electrons stored in the ferritin core tunnel to the QD.
Sensory processes
Olfaction
Olfaction, the sense of smell, can be broken down into two parts; the reception and detection of a chemical, and how that detection is sent to and processed by the brain. This process of detecting anodorant
An aroma compound, also known as an odorant, aroma, fragrance, flavoring or flavor, is a chemical compound that has a smell or odor. For an individual chemical or class of chemical compounds to impart a smell or fragrance, it must be sufficien ...
is still under question. One theory named the " shape theory of olfaction" suggests that certain olfactory receptors are triggered by certain shapes of chemicals and those receptors send a specific message to the brain. Another theory (based on quantum phenomena) suggests that the olfactory receptors detect the vibration of the molecules that reach them and the "smell" is due to different vibrational frequencies, this theory is aptly called the "vibration theory of olfaction."
The vibration theory of olfaction, created in 1938 by Malcolm Dyson but reinvigorated by Luca Turin in 1996, proposes that the mechanism for the sense of smell is due to G-protein receptors that detect molecular vibrations due to inelastic electron tunneling, tunneling where the electron loses energy, across molecules. In this process a molecule would fill a binding site with a G-protein receptor. After the binding of the chemical to the receptor, the chemical would then act as a bridge allowing for the electron to be transferred through the protein. As the electron transfers across what would otherwise have been a barrier, it loses energy due to the vibration of the newly-bound molecule to the receptor. This results in the ability to smell the molecule.
While the vibration theory has some experimental proof of concept, there have been multiple controversial results in experiments. In some experiments, animals are able to distinguish smells between molecules of different frequencies and same structure, while other experiments show that people are unaware of distinguishing smells due to distinct molecular frequencies.
Vision
Vision relies on quantized energy in order to convert light signals to an action potential in a process called phototransduction. In phototransduction, a photon interacts with achromophore
A chromophore is the part of a molecule responsible for its color. The word is derived .
The color that is seen by our eyes is that of the light not Absorption (electromagnetic radiation), absorbed by the reflecting object within a certain wavele ...
in a light receptor. The chromophore absorbs the photon and undergoes photoisomerization. This change in structure induces a change in the structure of the photo receptor and resulting signal transduction
Signal transduction is the process by which a chemical or physical signal is transmitted through a cell as a biochemical cascade, series of molecular events. Proteins responsible for detecting stimuli are generally termed receptor (biology), rece ...
pathways lead to a visual signal. However, the photoisomerization reaction occurs at a rapid rate, in under 200 femtoseconds, with high yield. Models suggest the use of quantum effects in shaping the ground state
The ground state of a quantum-mechanical system is its stationary state of lowest energy; the energy of the ground state is known as the zero-point energy of the system. An excited state is any state with energy greater than the ground state ...
and excited state
In quantum mechanics
Quantum mechanics is the fundamental physical Scientific theory, theory that describes the behavior of matter and of light; its unusual characteristics typically occur at and below the scale of atoms. Reprinted, Add ...
potentials in order to achieve this efficiency.
The sensor in the retina of the human eye is sensitive enough to detect a single photon. Single photon
A photon () is an elementary particle that is a quantum of the electromagnetic field, including electromagnetic radiation such as light and radio waves, and the force carrier for the electromagnetic force. Photons are massless particles that can ...
detection could lead to multiple different technologies. One area of development is in quantum communication and cryptography
Cryptography, or cryptology (from "hidden, secret"; and ''graphein'', "to write", or ''-logy, -logia'', "study", respectively), is the practice and study of techniques for secure communication in the presence of Adversary (cryptography), ...
. The idea is to use a biometric system to measure the eye using only a small number of points across the retina
The retina (; or retinas) is the innermost, photosensitivity, light-sensitive layer of tissue (biology), tissue of the eye of most vertebrates and some Mollusca, molluscs. The optics of the eye create a focus (optics), focused two-dimensional ...
with random flashes of photons that "read" the retina and identify the individual. This biometric system would only allow a certain individual with a specific retinal map to decode the message. This message can not be decoded by anyone else unless the eavesdropper were to guess the proper map or could read the retina of the intended recipient of the message.
Theoretical and mathematical evidence of an underlying quantum structure in human color perception has been presented by Michel Berthier and Edoardo Provenzi in a series of scientific articles. Notably, in their quantum formalism, the chromatic opposition phenomena proposed by Hering emerge naturally. Uncertainty principles for the perception of opposition have been predicted within this framework, which has so far demonstrated concrete applications in the removal of color cast in natural images caused by the presence of a non-neutral illuminant.
Energy transfer
Photosynthesis


 Photosynthesis refers to the biological process that photosynthetic cells use to synthesize organic compounds from inorganic starting materials using sunlight. What has been primarily implicated as exhibiting non-trivial quantum behaviors is the light reaction stage of photosynthesis. In this stage, photons are absorbed by the membrane-bound photosystems. Photosystems contain two major domains, the light-harvesting complex (antennae) and the reaction center. These antennae vary among organisms. For example, bacteria use circular aggregates of chlorophyll pigments, while plants use membrane-embedded protein and chlorophyll complexes. Regardless, photons are first captured by the antennae and passed on to the reaction-center complex. Various pigment-protein complexes, such as the FMO complex in green sulfur bacteria, are responsible for transferring energy from antennae to reaction site. The photon-driven excitation of the reaction-center complex mediates the oxidation and the reduction of the primary electron acceptor, a component of the reaction-center complex. Much like the
Photosynthesis refers to the biological process that photosynthetic cells use to synthesize organic compounds from inorganic starting materials using sunlight. What has been primarily implicated as exhibiting non-trivial quantum behaviors is the light reaction stage of photosynthesis. In this stage, photons are absorbed by the membrane-bound photosystems. Photosystems contain two major domains, the light-harvesting complex (antennae) and the reaction center. These antennae vary among organisms. For example, bacteria use circular aggregates of chlorophyll pigments, while plants use membrane-embedded protein and chlorophyll complexes. Regardless, photons are first captured by the antennae and passed on to the reaction-center complex. Various pigment-protein complexes, such as the FMO complex in green sulfur bacteria, are responsible for transferring energy from antennae to reaction site. The photon-driven excitation of the reaction-center complex mediates the oxidation and the reduction of the primary electron acceptor, a component of the reaction-center complex. Much like the electron transport chain
An electron transport chain (ETC) is a series of protein complexes and other molecules which transfer electrons from electron donors to electron acceptors via redox reactions (both reduction and oxidation occurring simultaneously) and couples th ...
of the mitochondria, a linear series of oxidations and reductions drives proton (H+) pumping across the thylakoid membrane, the development of a proton motive force, and energetic coupling to the synthesis of ATP.
Previous understandings of electron-excitation transference (EET) from light-harvesting antennae to the reaction center have relied on the Förster theory of incoherent EET, postulating weak electron coupling between chromophore
A chromophore is the part of a molecule responsible for its color. The word is derived .
The color that is seen by our eyes is that of the light not Absorption (electromagnetic radiation), absorbed by the reflecting object within a certain wavele ...
s and incoherent hopping from one to another. This theory has largely been disproven by FT electron spectroscopy experiments that show electron absorption and transfer with an efficiency of above 99%, which cannot be explained by classical mechanical models. Instead, as early as 1938, scientists theorized that quantum coherence was the mechanism for excitation-energy transfer. Indeed, the structure and nature of the photosystem places it in the quantum realm, with EET ranging from the femto- to nanosecond scale, covering sub-nanometer to nanometer distances. The effects of quantum coherence on EET in photosynthesis are best understood through state and process coherence. State coherence refers to the extent of individual superpositions of ground and excited states for quantum entities, such as exciton
An exciton is a bound state of an electron and an electron hole which are attracted to each other by the electrostatic Coulomb's law, Coulomb force resulting from their opposite charges. It is an electrically neutral quasiparticle regarded as ...
s. Process coherence, on the other hand, refers to the degree of coupling between multiple quantum entities and their evolution as either dominated by unitary or dissipative parts, which compete with one another. Both of these types of coherence are implicated in photosynthetic EET, where a exciton is coherently delocalized over several chromophores. This delocalization allows for the system to simultaneously explore several energy paths and use constructive and destructive interference to guide the path of the exciton's wave packet. It is presumed that natural selection has favored the most efficient path to the reaction center. Experimentally, the interaction between the different frequency wave packets, made possible by long-lived coherence, will produce quantum beats.
While quantum photosynthesis is still an emerging field, there have been many experimental results that support the quantum-coherence understanding of photosynthetic EET. A 2007 study claimed the identification of electronic quantum coherence at −196 °C (77 K). Another theoretical study from 2010 provided evidence that quantum coherence lives as long as 300 femtoseconds at biologically relevant temperatures (4 °C or 277 K). In that same year, experiments conducted on photosynthetic cryptophyte algae using two-dimensional photon echo spectroscopy yielded further confirmation for long-term quantum coherence. These studies suggest that, through evolution, nature has developed a way of protecting quantum coherence to enhance the efficiency of photosynthesis. However, critical follow-up studies question the interpretation of these results. Single-molecule spectroscopy now shows the quantum characteristics of photosynthesis without the interference of static disorder, and some studies use this method to assign reported signatures of electronic quantum coherence to nuclear dynamics occurring in chromophores. A number of proposals emerged to explain unexpectedly long coherence. According to one proposal, if each site within the complex feels its own environmental noise, the electron will not remain in any local minimum due to both quantum coherence and its thermal environment, but proceed to the reaction site via quantum walk
Quantum walks are quantum analogs of classical random walks. In contrast to the classical random walk, where the walker occupies definite states and the randomness arises due to stochastic transitions between states, in quantum walks randomness ...
s. Another proposal is that the rate of quantum coherence and electron tunneling create an energy sink that moves the electron to the reaction site quickly. Other work suggested that geometric symmetries in the complex may favor efficient energy transfer to the reaction center, mirroring perfect state transfer in quantum networks. Furthermore, experiments with artificial dye molecules cast doubts on the interpretation that quantum effects last any longer than one hundred femtoseconds.
In 2017, the first control experiment with the original FMO protein under ambient conditions confirmed that electronic quantum effects are washed out within 60 femtoseconds, while the overall exciton transfer takes a time on the order of a few picoseconds. In 2020 a review based on a wide collection of control experiments and theory concluded that the proposed quantum effects as long lived electronic coherences in the FMO system does not hold. Instead, research investigating transport dynamics suggests that interactions between electronic and vibrational modes of excitation in FMO complexes require a semi-classical, semi-quantum explanation for the transfer of exciton energy. In other words, while quantum coherence dominates in the short-term, a classical description is most accurate to describe long-term behavior of the excitons.
Another process in photosynthesis that has almost 100% efficiency is charge transfer, again suggesting that quantum mechanical phenomena are at play. In 1966, a study on the photosynthetic bacterium Chromatium found that at temperatures below 100 K, cytochrome
Cytochromes are redox-active proteins containing a heme, with a central iron (Fe) atom at its core, as a cofactor. They are involved in the electron transport chain and redox catalysis. They are classified according to the type of heme and its ...
oxidation is temperature-independent, slow (on the order of milliseconds), and very low in activation energy
In the Arrhenius model of reaction rates, activation energy is the minimum amount of energy that must be available to reactants for a chemical reaction to occur. The activation energy (''E''a) of a reaction is measured in kilojoules per mole (k ...
. The authors, Don DeVault and Britton Chase, postulated that these characteristics of electron transfer are indicative of quantum tunneling
In physics, a quantum (: quanta) is the minimum amount of any physical entity (physical property) involved in an interaction. The fundamental notion that a property can be "quantized" is referred to as "the hypothesis of quantization". This me ...
, whereby electrons penetrate a potential barrier despite possessing less energy than is classically necessary.
Mitochondria
Mitochondria
A mitochondrion () is an organelle found in the cells of most eukaryotes, such as animals, plants and fungi. Mitochondria have a double membrane structure and use aerobic respiration to generate adenosine triphosphate (ATP), which is us ...
have been demonstrated to utilize quantum tunneling
In physics, a quantum (: quanta) is the minimum amount of any physical entity (physical property) involved in an interaction. The fundamental notion that a property can be "quantized" is referred to as "the hypothesis of quantization". This me ...
in their function as the powerhouse of eukaryotic cells. Similar to the light reactions in the thylakoid
Thylakoids are membrane-bound compartments inside chloroplasts and cyanobacterium, cyanobacteria. They are the site of the light-dependent reactions of photosynthesis. Thylakoids consist of a #Membrane, thylakoid membrane surrounding a #Lumen, ...
, linearly-associated membrane-bound proteins comprising the electron transport chain
An electron transport chain (ETC) is a series of protein complexes and other molecules which transfer electrons from electron donors to electron acceptors via redox reactions (both reduction and oxidation occurring simultaneously) and couples th ...
(ETC) energetically link the reduction of O2 with the development of a proton motive gradient (H+) across the inner membrane of the mitochondria. This energy stored as a proton motive gradient is then coupled with the synthesis of ATP. It is significant that the mitochondrion conversion of biomass into chemical ATP achieves 60-70% thermodynamic efficiency, far superior to that of man-made engines. This high degree of efficiency is largely attributed to the quantum tunnelling of electrons in the ETC and of protons in the proton motive gradient. Indeed, electron tunneling has already been demonstrated in certain elements of the ETC including NADH:ubiquinone oxidoreductase(Complex I) and CoQH2-cytochrome c reductase (Complex III).
In quantum mechanics, both electrons and protons are quantum entities that exhibit wave-particle duality, exhibiting both particle and wave-like properties depending on the method of experimental observation. Quantum tunneling is a direct consequence of this wave-like nature of quantum entities that permits the passing-through of a potential energy barrier that would otherwise restrict the entity. Moreover, it depends on the shape and size of a potential barrier relative to the incoming energy of a particle. Because the incoming particle is defined by its wave function, its tunneling probability is dependent upon the potential barrier's shape in an exponential way. For example, if the barrier is relatively wide, the incoming particle's probability to tunnel will decrease. The potential barrier, in some sense, can come in the form of an actual biomaterial barrier. The inner mitochondria membrane which houses the various components of the ETC is on the order of 7.5 nm thick. The inner membrane of a mitochondrion must be overcome to permit signals (in the form of electrons, protons, H+) to transfer from the site of emittance (internal to the mitochondria) and the site of acceptance (i.e. the electron transport chain proteins). In order to transfer particles, the membrane of the mitochondria must have the correct density of phospholipids to conduct a relevant charge distribution that attracts the particle in question. For instance, for a greater density of phospholipids, the membrane contributes to a greater conductance of protons.
Molecular solitons in proteins
Alexander Davydov developed the quantum theory of molecularsoliton
In mathematics and physics, a soliton is a nonlinear, self-reinforcing, localized wave packet that is , in that it preserves its shape while propagating freely, at constant velocity, and recovers it even after collisions with other such local ...
s in order to explain the transport of energy in protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
α-helices in general and the physiology
Physiology (; ) is the science, scientific study of function (biology), functions and mechanism (biology), mechanisms in a life, living system. As a branches of science, subdiscipline of biology, physiology focuses on how organisms, organ syst ...
of muscle contraction
Muscle contraction is the activation of Tension (physics), tension-generating sites within muscle cells. In physiology, muscle contraction does not necessarily mean muscle shortening because muscle tension can be produced without changes in musc ...
in particular. He showed that the molecular solitons are able to preserve their shape through nonlinear interaction of amide I exciton
An exciton is a bound state of an electron and an electron hole which are attracted to each other by the electrostatic Coulomb's law, Coulomb force resulting from their opposite charges. It is an electrically neutral quasiparticle regarded as ...
s and phonon
A phonon is a collective excitation in a periodic, elastic arrangement of atoms or molecules in condensed matter, specifically in solids and some liquids. In the context of optically trapped objects, the quantized vibration mode can be defined a ...
deformations inside the lattice of hydrogen-bond
In chemistry, a hydrogen bond (H-bond) is a specific type of molecular interaction that exhibits partial covalent character and cannot be described as a purely electrostatic force. It occurs when a hydrogen (H) atom, covalently bonded to a mo ...
ed peptide groups. In 1979, Davydov published his complete textbook on quantum biology entitled "Biology and Quantum Mechanics" featuring quantum dynamics of protein
Proteins are large biomolecules and macromolecules that comprise one or more long chains of amino acid residue (biochemistry), residues. Proteins perform a vast array of functions within organisms, including Enzyme catalysis, catalysing metab ...
s, cell membrane
The cell membrane (also known as the plasma membrane or cytoplasmic membrane, and historically referred to as the plasmalemma) is a biological membrane that separates and protects the interior of a cell from the outside environment (the extr ...
s, bioenergetics, muscle contraction
Muscle contraction is the activation of Tension (physics), tension-generating sites within muscle cells. In physiology, muscle contraction does not necessarily mean muscle shortening because muscle tension can be produced without changes in musc ...
, and electron
The electron (, or in nuclear reactions) is a subatomic particle with a negative one elementary charge, elementary electric charge. It is a fundamental particle that comprises the ordinary matter that makes up the universe, along with up qua ...
transport
Transport (in British English) or transportation (in American English) is the intentional Motion, movement of humans, animals, and cargo, goods from one location to another. Mode of transport, Modes of transport include aviation, air, land tr ...
in biomolecule
A biomolecule or biological molecule is loosely defined as a molecule produced by a living organism and essential to one or more typically biological processes. Biomolecules include large macromolecules such as proteins, carbohydrates, lipids ...
s.
Information encoding
Magnetoreception
spin chemistry
Spin chemistry is a sub-field of chemistry positioned at the intersection of chemical kinetics, photochemistry, Nuclear magnetic resonance, magnetic resonance and free radical chemistry, that deals with magnetic and Spin (physics), spin effects in ...
, and was speculated to apply to magnetoreception in 1978 by Schulten et al.. The ratio between singlet and triplet pairs is changed by the interaction of entangled electron pairs with the magnetic field of the Earth. In 2000, cryptochrome was proposed as the "magnetic molecule" that could harbor magnetically sensitive radical-pairs. Cryptochrome, a flavoprotein
Flavoproteins are proteins that contain a nucleic acid derivative of riboflavin. These proteins are involved in a wide array of biological processes, including removal of radicals contributing to oxidative stress, photosynthesis, and DNA repair. ...
found in the eyes of European robins and other animal species, is the only protein known to form photoinduced radical-pairs in animals. When it interacts with light particles, cryptochrome goes through a redox
Redox ( , , reduction–oxidation or oxidation–reduction) is a type of chemical reaction in which the oxidation states of the reactants change. Oxidation is the loss of electrons or an increase in the oxidation state, while reduction is t ...
reaction, which yields radical pairs both during the photo-reduction and the oxidation. The function of cryptochrome is diverse across species, however, the photoinduction of radical-pairs occurs by exposure to blue light, which excites an electron in a chromophore
A chromophore is the part of a molecule responsible for its color. The word is derived .
The color that is seen by our eyes is that of the light not Absorption (electromagnetic radiation), absorbed by the reflecting object within a certain wavele ...
. Magnetoreception is also possible in the dark, so the mechanism must rely more on the radical pairs generated during light-independent oxidation.
Experiments in the lab support the basic theory that radical-pair electrons can be significantly influenced by very weak magnetic fields, i.e., merely the direction of weak magnetic fields can affect radical-pair's reactivity and therefore can "catalyze" the formation of chemical products. Whether this mechanism applies to magnetoreception and/or quantum biology, that is, whether Earth's magnetic field "catalyzes" the formation of ''bio''chemical products by the aid of radical-pairs, is not fully clear. Radical-pairs may need not be entangled, the key ''quantum'' feature of the radical-pair mechanism, to play a part in these processes. There are entangled and non-entangled radical-pairs, but disturbing only entangled radical-pairs is not possible with current technology. Researchers found evidence for the radical-pair mechanism of magnetoreception when European robins, cockroaches, and garden warblers, could no longer navigate when exposed to a radio frequency
Radio frequency (RF) is the oscillation rate of an alternating electric current or voltage or of a magnetic, electric or electromagnetic field or mechanical system in the frequency range from around to around . This is roughly between the u ...
that obstructs magnetic field
A magnetic field (sometimes called B-field) is a physical field that describes the magnetic influence on moving electric charges, electric currents, and magnetic materials. A moving charge in a magnetic field experiences a force perpendicular ...
s and radical-pair chemistry. Further evidence came from a comparison of Cryptochrome 4 (CRY4) from migrating and non-migrating birds. CRY4 from chicken and pigeon were found to be less sensitive to magnetic fields than those from the (migrating) European robin, suggesting evolutionary optimization of this protein as a sensor of magnetic fields.
DNA mutation
DNA
Deoxyribonucleic acid (; DNA) is a polymer composed of two polynucleotide chains that coil around each other to form a double helix. The polymer carries genetic instructions for the development, functioning, growth and reproduction of al ...
acts as the instructions for making proteins throughout the body. It consists of 4 nucleotides: guanine, thymine, cytosine, and adenine. The order of these nucleotides gives the "recipe" for the different proteins.
Whenever a cell reproduces, it must copy these strands of DNA. However, sometime throughout the process of copying the strand of DNA a mutation, or an error in the DNA code, can occur. A theory for the reasoning behind DNA mutation is explained in the Lowdin DNA mutation model. In this model, a nucleotide may spontaneously change its form through a process of quantum tunneling
In physics, a quantum (: quanta) is the minimum amount of any physical entity (physical property) involved in an interaction. The fundamental notion that a property can be "quantized" is referred to as "the hypothesis of quantization". This me ...
. Because of this, the changed nucleotide will lose its ability to pair with its original base pair and consequently change the structure and order of the DNA strand.
Exposure to ultraviolet light and other types of radiation can cause DNA mutation and damage. The radiation also can modify the bonds along the DNA strand in the pyrimidines and cause them to bond with themselves, creating a dimer.
In many prokaryotes and plants, these bonds are repaired by a DNA-repair-enzyme photolyase. As its prefix implies, photolyase is reliant on light in order to repair the strand. Photolyase works with its cofactor FADH, flavin adenine dinucleotide, while repairing the DNA. Photolyase is excited by visible light and transfers an electron to the cofactor FADH. FADH—now in the possession of an extra electron—transfers the electron to the dimer to break the bond and repair the DNA. The electron tunnels from the FADH to the dimer. Although the range of this tunneling is much larger than feasible in a vacuum, the tunneling in this scenario is said to be "superexchange-mediated tunneling," and is possible due to the protein's ability to boost the tunneling rates of the electron.
Other
Other quantum phenomena in biological systems include the conversion ofchemical energy
Chemical energy is the energy of chemical substances that is released when the substances undergo a chemical reaction and transform into other substances. Some examples of storage media of chemical energy include batteries, Schmidt-Rohr, K. (20 ...
into motion and brownian motors in many cellular processes.
Pseudoscience
Alongside the multiple strands of scientific inquiry into quantum mechanics has come unconnectedpseudoscientific
Pseudoscience consists of statements, beliefs, or practices that claim to be both scientific and factual but are incompatible with the scientific method. Pseudoscience is often characterized by contradictory, exaggerated or unfalsifiable cl ...
interest; this caused scientists to approach quantum biology cautiously.
Hypotheses such as orchestrated objective reduction which postulate a link between quantum mechanics and consciousness
Consciousness, at its simplest, is awareness of a state or object, either internal to oneself or in one's external environment. However, its nature has led to millennia of analyses, explanations, and debate among philosophers, scientists, an ...
have drawn criticism from the scientific community with some claiming it to be pseudoscientific and "an excuse for quackery".
References
External links
Philip Ball (2015). "Quantum Biology: An Introduction". The Royal Institution
Quantum Biology and the Hidden Nature of Nature, World Science Festival 2012, video of podium discussion
Quantum Biology: Current Status and Opportunities, September 17-18, 2012, University of Surrey, UK
{{DEFAULTSORT:Quantum Biology Biophysics